$25 Million Boosts Clinical Trials Conducted at Pharmacies

When the U.S. government approves a vaccine, the clinical trial process does not stop. The fourth type of clinical evaluation is called Phase 4 or Post-Market Survalence.
These studies aggregate real-world evidence that hopefully meets or extends results reported in the pre-approval clinical trial.
To increase access to underfunded phase 4 clinical trials, the U.S. Administration for Strategic Preparedness and Response’s Biomedical Advanced Research and Development Authority (BARDA) announced that it would provide $25 million in Project NextGen funding to Walgreens Inc. to conduct a decentralized phase four clinical trial.
To date, BARDA has provided approximately $2.6 billion in Project NextGen funds to industry partners.
The new agreement is BARDA’s first with a major retail pharmacy chain with about 8,500 locations to use this type of clinical trial approach, which is expected to enable more people of diverse backgrounds, such as age and ethnicity, to participate.
Currently, most clinical trial volunteers often travel to hospitals, universities, or other locations that are usually far from their homes. Decentralized settings include healthcare environments that Americans routinely use.
For this new study, BARDA is leveraging certified pharmacists throughout the U.S. who patients highly trust. Approximately 20 Walgreens pharmacies will serve as clinical trial sites in urban, suburban, and rural communities across the U.S.
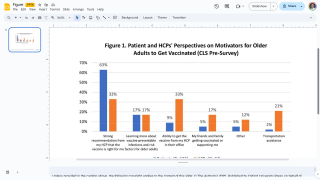
During the recent pandemic, pharmacies and retail clinics were essential for distributing vaccines, therapeutics, and diagnostic tests. Many Americans received at least one vaccination at a retail pharmacy.
“Today’s announcement is the latest demonstration of our commitment to ensuring that health equity is built into all of our preparedness, response, and recovery activities,” said Assistant Secretary for Preparedness and Response Dawn O’Connell in a press release on July 23, 2024.
"Americans are diverse. Clinical trials should be, too. We want to make clinical trials easy to access so that more people who want to participate can do so.”
Under the BARDA agreement, Walgreens will conduct a Phase 4 vaccine correlate of protection clinical study using a U.S. FDA-authorized or licensed COVID-19 vaccine.
Correlates of protection, also known as correlates of immunity, are measurable markers of immunity that correlate with protection against disease. Establishing correlates helps scientists and others understand more about how the protective immunity of vaccines lasts over time, which informs public health guidelines.
Correlates of protection also help scientists evaluate vaccines, providing information on how to improve vaccines, potentially saving time and reducing the cost of developing future vaccines through clinical trials.
Should this new initiative succeed, this innovative tactic may be deployed for various U.S. FDA-approved vaccines.
This effort is being funded under Project NextGen, a $5 billion program led by BARDA and others to accelerate and streamline the development of the next generation of innovative COVID-19 vaccines, therapeutics, and enabling technologies.
Our Trust Standards: Medical Advisory Committee