Novavax Earns $50 Million from Sanofi Partnership

Novavax, Inc. today announced progress in advancing its corporate growth strategy through its partnership with Sanofi, a Paris, France based company.
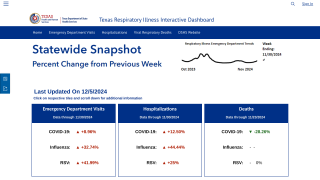
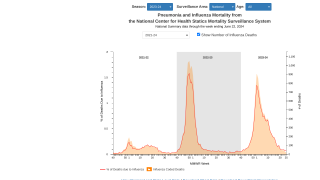
Novavax has achieved a milestone associated with its Phase 2/3 clinical trial for its COVID-19 vaccine in children, triggering Sanofi's first $50 million milestone payment.
Novavax's COVID-19 vaccine is included in Sanofi's two combination vaccine candidates for the prevention of influenza and COVID-19, for which Phase 1/2 trials were initiated, and the U.S. FDA Fast Track designation was recently granted in the U.S.
n addition to milestones for the stand-alone COVID-19 vaccine, the agreement also includes combination products developed by Sanofi including Novavax's COVID-19 vaccine, which present a potential opportunity of up to an additional $350 million for Novavax in future milestones.
In addition, stand-alone COVID-19 sales and potential sales of any Sanofi combination products would net Novavax ongoing tiered royalties.
Further, Novavax is eligible to receive up to $200 million for the first four products created by Sanofi utilizing its Matrix-M adjuvant and up to $210 million in milestone payments for each product, including Matrix-M thereafter, plus ongoing royalties for all Sanofi products utilizing Matrix-M.
Our Trust Standards: Medical Advisory Committee