Chikungunya Vaccination Induces Sustained Protective Antibody Concentrations

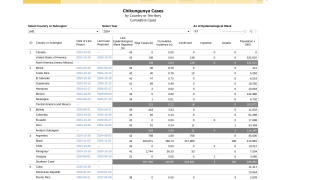
Over the past decade, Chikungunya virus disease has become one of the most neglected arboviral diseases transmitted by infected mosquitoes. During 2024, Chikungunya outbreaks were confirmed throughout the Region of the Americas, infecting over 412,000 people.
While Chikungunya does not often result in death (204 people in 2024), the joint pain associated with the disease may last for months or years and may become a cause of chronic pain and disability.
Unlike previous years, a highly effective U.S. FDA-approved vaccine is commercially available in the United States and various other countries.
To quantify the effectiveness of Valneva SE's IXCHIQ® (VLA1553) monovalent, single-dose vaccine, The Lancet Infectious Diseases, Robert McMahon and colleagues reported in December (Volume 24, Issue 12, p1298-1299) their single-arm, multicentre, phase 3b clinical trial assessing antibody persistence and safety up to 2 years after a single vaccination with the chikungunya virus vaccine.
Valneva announced on December 3, 2024, that among healthy adults still enrolled in the trial, 96% maintained neutralizing antibody titers well above the seroresponse threshold three years after the single-dose vaccination.
The U.S. CDC encourages international travelers to speak with a travel vaccine expert at least one month before visiting Chikungunya-endemic areas in 2024.
Our Trust Standards: Medical Advisory Committee