Chikungunya Vaccine Significantly Protects People for Three Years

Valneva SE today reported positive antibody persistence data three years after vaccination with a single dose of its chikungunya vaccine IXCHIQ®.
Among the healthy adults still enrolled in a clinical trial, 96% maintained neutralizing antibody titers.
Announced on December 3, 2024, the results align with Valneva's expectations for the only U.S. FDA-approved chikungunya vaccine, confirming a strong and long-lasting antibody persistence across all age groups investigated.
IXCHIQ's three-year persistence data also align with positive twelve-month and two-year persistence data the Company reported in December 2022 and 2023, respectively.
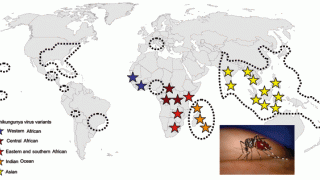
These data are positive news for the Region of the Americas, where 412,094 mosquito-transmitted chikungunya cases and 204 related fatalities have been reported this year.
In the United States, the Centers for Disease Control and Prevention reported 173 travel-related chikungunya cases in Territories and non-U.S. residents in 2024, led by Massachusetts (20) and Texas (20).
Juan Carlos Jaramillo, M.D., Chief Medical Officer of Valneva, commented in a press release, "We are extremely pleased about these three-year data, which further highlight IXCHIQ®'s differentiated product profile and ability to induce a robust, long-lasting antibody response in both younger and older adults with a single vaccination."
"Whether you're a traveler or live in an endemic region, the potential for long-term protection against a mosquito-borne disease with a single dose is crucial, particularly in low- and middle-income countries where vaccine access is often limited."
IXCHIQ® is the world's first and only licensed chikungunya vaccine approved in the U.S., Europe, and Canada to address this significant unmet medical need.
The Company expects a marketing authorization in Brazil, a chikungunya hot spot, before the end of 2024.
In the U.S., IXCHIQ is available at travel clinics and pharmacies, such as Passport Health in Tampa, Florida.
Our Trust Standards: Medical Advisory Committee