Investigational Antiviral for the Prevention of Dengue Discontinued

When Johnson & Johnson (J&J) announced third-quarter earnings this week, its growth exceeded Wall Street’s expectations.
However, based on J&J's announcement on October 4, 2024, its future results will not include a Dengue product.
J&J confirmed the discontinuance of the Phase 2 field study to evaluate the efficacy of the investigational antiviral candidate mosnodenvir in preventing Dengue virus in adults. The decision to discontinue this study is part of a strategic reprioritization of the Company’s Communicable Diseases research and development portfolio.
This unfortunate news will bring essential focus to second-generation dengue vaccines and innovative vaccine candidates conducting late-stage clinical research in 2024, such as Butantan Institute's Butantan-DV tetravalent dengue vaccine.
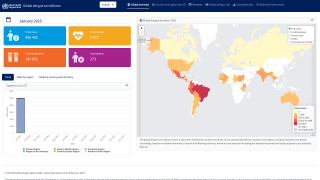
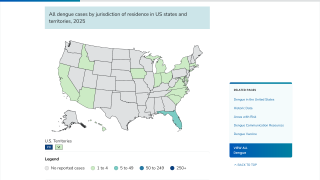
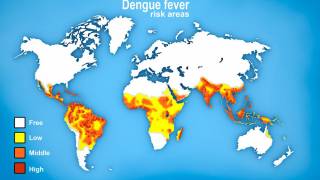
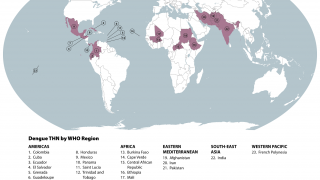
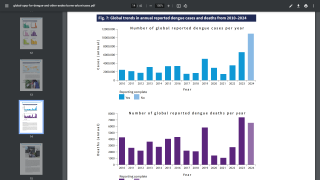
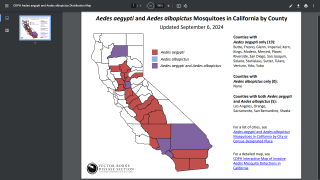
As of October 18, 2024, Dengue's global outbreak continues unabated.
Our Trust Standards: Medical Advisory Committee