Phase I Data Confirms Immunogenicity of Adjuvant Treatment of Head and Neck Cancers
Transgene and NEC Corporation today announced that new data will be presented on TG4050, an individualized neoantigen cancer vaccine, at the American Association for Cancer Research (AACR) Annual Meeting.
TG4050 is being evaluated in a randomized multicenter Phase I/II trial as a single agent in the adjuvant treatment of HPV-negative head and neck cancers.
Key findings of the poster presentation obtained in the Phase I part of the trial (NCT04183166) include, but are not limited to, the following:
All 16 patients who received TG4050 are disease-free after a median 18.6-month follow-up. Out of the 16 patients in the control observation arm, three patients have relapsed. For this head and neck cancer patient population and with the current standard of care (chemoradiotherapy), approximately 40% of patients are expected to relapse within 24 months following surgery and adjuvant therapy. Also, the tumor immune contexture, expression of immune factors, mutational burden, and tumor infiltrates are associated with challenging prognoses.
Specific cellular immune responses were detected in the 16/17 patients who received TG4050 (16 patients from the treatment arm and one from the observation arm treated after relapse) using stringent testing conditions.
TG4050 induced persistent immune responses against multiple targets in several patients. T-cell responses were maintained beyond 211 days (7 months) after the initiation of the treatment.
Dr Oliver Lantz, Head of the clinical immunology laboratory at Institut Curie, commented in the April 9, 2024, press release, "The immunological data generated by TG4050 demonstrate a robust and specific cellular immune response, even under stringent measurement criteria."
"The diversity, depth, and duration of these responses were most certainly a key factor in preventing relapse in the patients treated with TG4050."
According to statements, Transgene and NEC are preparing a randomized Phase II extension of this trial, slated to start in the second quarter of 2024.
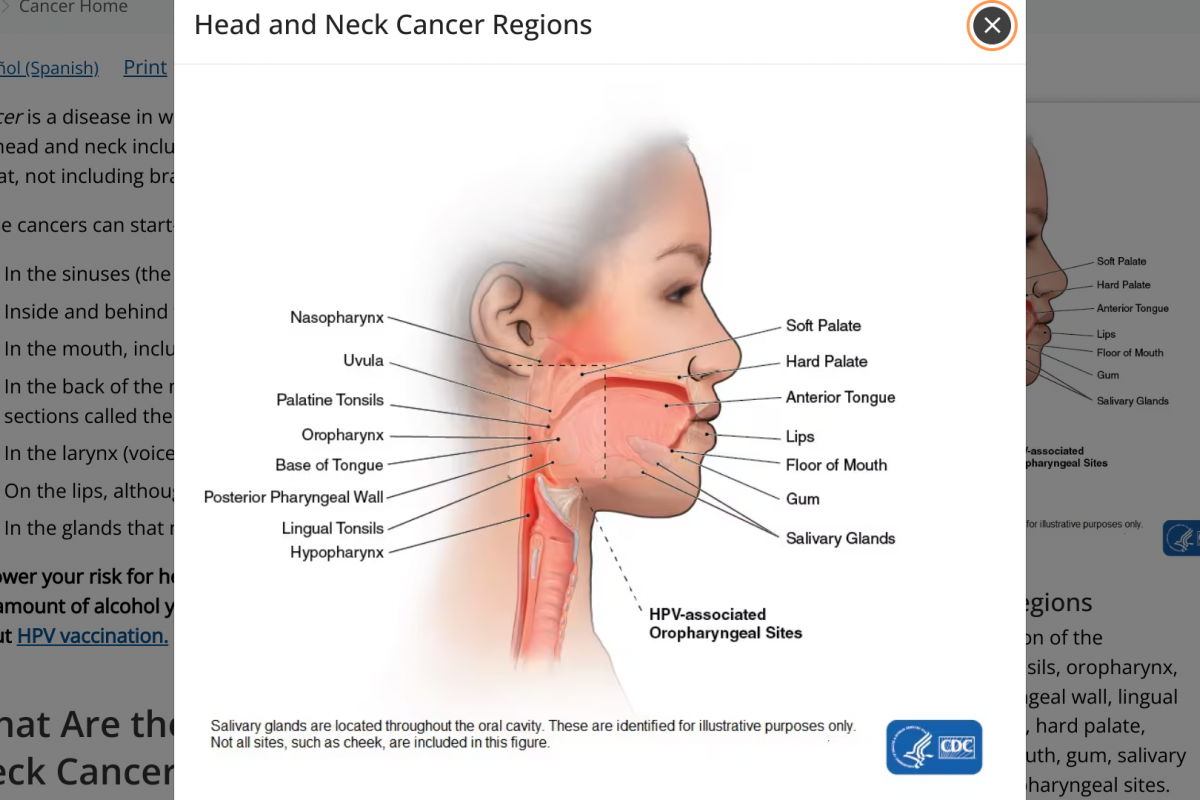
The U.S. CDC says cancers of the head and neck include cancers that start in several places in the head and throat. Cancer is a disease in which cells of the body grow out of control.
About 70% of cancers in the oropharynx (which includes the tonsils, soft palate, and base of the tongue) are linked to human papillomavirus, a common sexually transmitted virus, says the CDC.
Our Trust Standards: Medical Advisory Committee