Dengue Vaccine Booster Doses Found Non-Inferior
The peer-review journal The Lancet Infectious Diseases recently published the results of a phase 2 clinical study that assessed the need for a dengue vaccine booster after the primary vaccination regimens.
These researchers found that a Dengvaxia (CYD-TDV) vaccine booster 1 or 2 years after the two-dose or three-dose primary regimen does not elicit a consistent, meaningful booster effect against all dengue serotypes in participants who are seropositive for dengue at baseline.
And no safety concerns occurred with the 1-year or 2-year Dengvaxia booster.
Thus, non-inferiority of the 1-year or 2-year booster was not shown in dengue-seropositive people.
Dengue is caused by four closely related viruses (DENV-1–4), and a person can be infected with each serotype for a total of four infections during their lifetime.
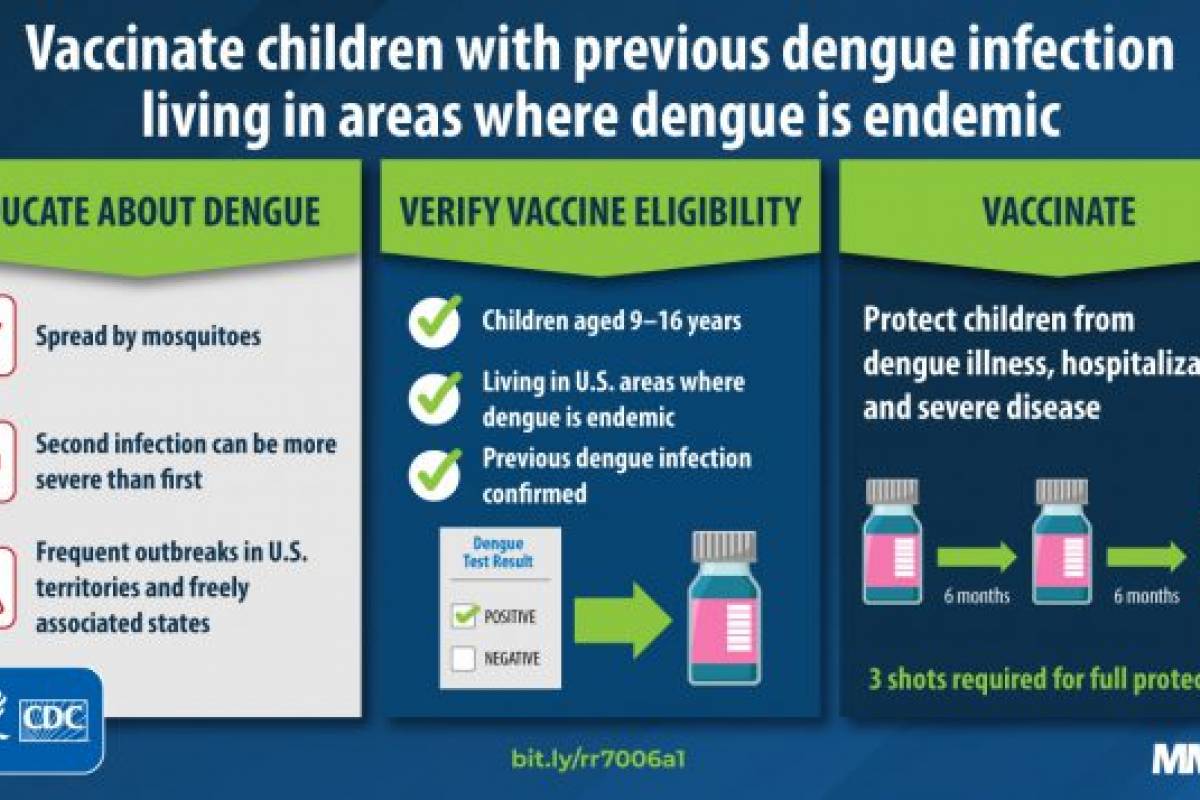
The U.S. CDC recommends vaccination with Dengvaxia for children aged 9–16 having evidence of previous dengue infection and living in areas where dengue is endemic.
Evidence of previous dengue infection, such as detection of anti-DENV immunoglobulin G with a highly specific serodiagnostic test, is required for eligible children before vaccination.
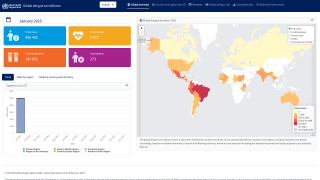
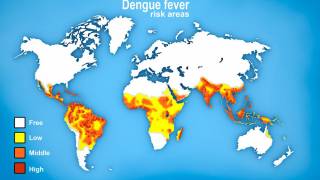
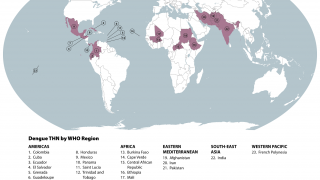
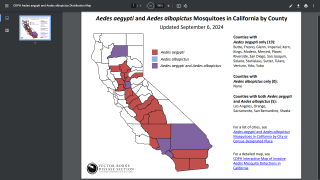
Dengue is a vector-borne infectious disease caused by dengue viruses (DENVs), predominantly transmitted by Aedes aegypti and Aedes albopictus mosquitos in many countries throughout the tropics and subtropics.
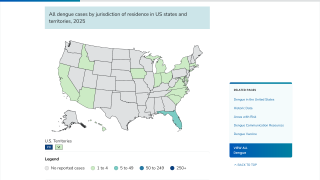
Areas where dengue is endemic in the U.S. and its territories and freely associated states include Puerto Rico, American Samoa, the U.S. Virgin Islands, the Federated States of Micronesia, the Republic of Marshall Islands, and the Republic of Palau.
During 2010–2020, approximately 95% of locally acquired dengue cases in the U.S. (31,289) occurred in Puerto Rico (29,779).
A previous, unrelated study published in July 2021 found 'at a base case cost of vaccination of $382, an incremental cost-effectiveness ratio of $122,000 per quality-adjusted life-year gained from Dengvaxia vaccination and that 5.5% of dengue hospitalizations in Puerto Rico could be averted.
The vaccine's producer, Sanofi Pasteur, funded this study.
Additional dengue vaccine news is posted at Vax-Before-Travel.
Note: This study and CDC content were edited for clarity and manually curated for mobile readers.
Our Trust Standards: Medical Advisory Committee