US FDA Approves IL-15 Superagonist for Treating Bladder Cancer
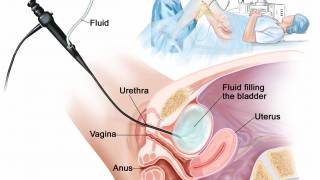
For several decades, a century-old vaccine has been used to treat a specific type of bladder cancer. In the 1970s, the Bacillus Calmette-Guérin (BCG) vaccine was approved as an immunotherapeutic treatment for bladder cancer patients.
Since then, various BCG vaccines have become the standard therapy for treating high-risk nonmuscle-invasive bladder cancer (NMIBC) patients to prevent the recurrence and progression of the disease.
Although BCG has shown moderate efficacy, researchers have sought a better therapy for bladder cancer patients.
Effective today, the U.S. Food and Drug Administration (FDA) has approved a new drug called Anktiva™ (nogapendekin alfa inbakicept-pmln) combined with Merck's TICE BCG for the treatment of adults with BCG-unresponsive NMIBC that has carcinoma in situ (CIS), with or without papillary tumors.
With the FDA's approval of Anktiva in combination with BCG, NMIBC patients who would otherwise face highly invasive surgery with life-long consequences have an important new therapeutic option with a long-term, durable, complete response, says ImmunityBio, Inc.
"The FDA's approval of Anktiva marks our launch of a next-generation immunotherapy beyond checkpoint inhibitors," said Patrick Soon-Shiong, M.D., Executive Chairman and Global Chief Scientific and Medical Officer at ImmunityBio, in a press release on April 22, 2024.
"Anktiva not only proliferates and activates the patient's own NK cells and CD8+ killer T cells, but also activates CD4+ T helper cells, thus enhancing the proliferation of memory killer T cells."
"This novel mechanism of action, which mimics the biology of the dendritic cell, begins the evolution of immunotherapy beyond T cells alone."
Dr. Soon-Shiong added, "We believe that by orchestrating the innate and adaptive immune system and driving long-term complete remission, ANKTIVA has the potential to play a key role as the immunotherapy beyond checkpoints in multiple tumor types in the years to come."
Bladder cancer is the 10th most commonly diagnosed cancer, and in the U.S., the American Cancer Society estimates there will be 83,190 new cases and 16,840 deaths from bladder cancer in 2024.
The annual projected total cost of bladder cancer treatment is over $2 billion.
The cost profile differs substantially among the stages of the disease. The average cost per patient varied from $19,521 (stage 0a) to $169,533 (metastatic disease).
According to the Compnay, Anktiva will be priced at $38,500 per dose.
According to the Company, Anktiva is expected to be available in the U.S. by mid-May 2024. ImmunityBio's Patient Assistance Program is expected to become operational for qualifying patients in May.
Our Trust Standards: Medical Advisory Committee