Non-Muscle-Invasive Bladder Cancer Vaccine Candidate Competes Resubmission to US FDA

When the U.S. Food and Drug Administration (FDA) issued a complete response letter in May 2023, it indicated that the agency could not approve the biologics license application (BLA) for a Non-Muscle-Invasive Bladder Cancer (NMIBC) vaccine candidate, thousands of people impacted by this common cancer were disappointed.
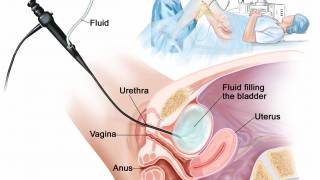
However, ImmunityBio, Inc. today announced it has completed the resubmission of its BLA to the FDA for N-803 (Anktiva®), a first-in-class IL-15 superagonist, plus Bacillus Calmette-Guérin (BCG) for the treatment of BCG-unresponsive NMIBC carcinoma in situ (CIS) with or without Ta or T1 disease.
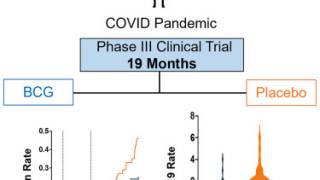
On October 23, 2023, the Company confirmed as part of this FDA resubmission, ImmunityBio provided an update on the Duration of Complete Response (CR) in BCG-unresponsive NMIBC patients with CIS ± Ta/T1 disease demonstrating a prolonged duration of remission with a median duration of CR not yet reached with a follow-up in responders exceeding 28 months.
In the responding BCG-unresponsive NMIBC patients, updated efficacy data demonstrated a probability of avoiding a cystectomy at ≥ 24 months of over 90%.
ImmunityBio also provided an update on the long-term follow-up clinical trial in BCG Naïve NMIBC patients, demonstrating a prolonged duration of complete remission in 6 out of 6 patients available for follow-up with a median survival of 8.8 years with ongoing bladder preservation to date.
These findings in both BCG-unresponsive and BCG-naïve patients demonstrate a prolonged duration of complete remission exceeding two years in the unresponsive cohort and over eight years in the BCG-naïve cohort.
This is positive news as bladder cancer is the fourth most common cancer in men, but it's less common in women, says the American Cancer Society (ACS). About 82,290 new bladder cancer cases (about 62,420 in men) are diagnosed annually.
As of October, the FDA has not approved a vaccine targeting this type of bladder cancer, and treatment options for BCG-unresponsive NMIBC patients are limited, says the ACS.
Patients with intermediate or high-risk NMIBC typically receive a treatment of transurethral resection of the bladder tumor (TURBT) followed by BCG intravesical instillation.
However, cancer will recur in 30% to 40% of patients with NMIBC despite adequate treatment with BCG.
Moreover, even among those in whom a complete response is achieved with BCG, up to 50% see their cancer return.
Recently, ImmunityBio announced that it has executed financing transactions resulting in approximately $200 million of proceeds to the Company.
As of September 11, 2023, ImmunityBio believes that it is well-positioned to fund its ongoing business operations and pre-commercialization efforts as it continues to drive toward potential regulatory approval of N-803 plus BCG for BCG-unresponsive non-muscle invasive bladder cancer.
Richard Adcock, Chief Executive Officer and President of ImmunityBio commented in a related press release, "With this additional financing, we are well positioned to execute our commercialization plans in anticipation of the approval of N-803 plus BCG in bladder cancer."
"This funding will also help support the planned expansion of our current clinical trials and the opening of new studies to explore the untapped potential of N-803 and our other platforms across multiple indications."
N-803 is a novel investigational IL-15 superagonist complex with an IL-15 mutant (IL-15N72D) bound to an IL-15 receptor α/IgG1 Fc fusion protein. The cytokine IL-15 plays a crucial role in the immune system by affecting the natural killer and T cells' development, maintenance, and function.
Merck's TICE® BCG vaccine is used in this therapy.
The N-803 vaccine candidate previously received both Breakthrough Therapy and Fast Track designations by the FDA for the treatment of BCG-unresponsive NMIBC CIS, as well as Fast Track designation for BCG-unresponsive NMIBC papillary and BCG-naïve NMIBC CIS.
Our Trust Standards: Medical Advisory Committee