Bladder Cancer Immunotherapy First Commercial Doses Administered
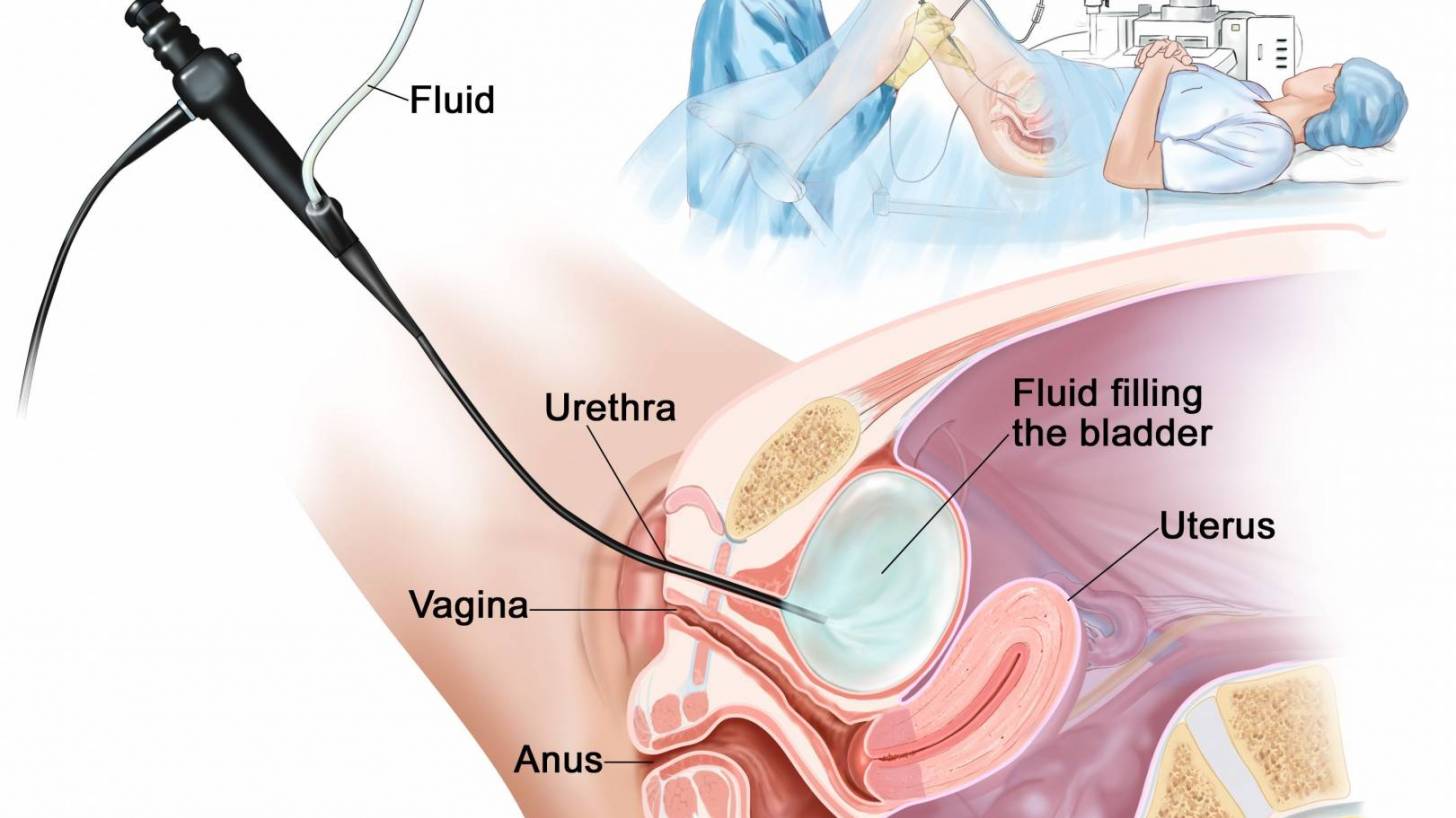
Over the past two months, people confronting a specific type of bladder cancer have received some optimistic news.
Multiple cancer patients have been treated with ANKTIVA® less than eight weeks after the U.S. FDA approved ImmunityBio Inc.’s approved immunotherapy for Bacillus Calmette-Guérin (BCG)-unresponsive non-muscle invasive bladder cancer (NMIBC) carcinoma in situ.
On June 20, 2024, ImmunityBio confirmed the initial treatments with ANKTIVA in multiple patients in the United States. The company previously announced that ANKTIVA Drug Substance was released with two-year storage stability data sufficient for 170,000 doses.
And urologists treating eligible bladder cancer patients in the U.S. can now access the ImmunityBio CARE™ support program, which supports healthcare professionals and patients through comprehensive resources, from benefits investigation to billing assistance.
“We are pleased to see interest in our bladder cancer therapeutic among healthcare providers and are encouraged by the number of health plans covering this first-in-class therapy. These updates underscore the importance of a new treatment option for this difficult-to-treat cancer, and we’re actively working to increase access to this treatment for as many patients as we can," wrote Richard Adcock, President & CEO of ImmunityBio, in a message to Precision Vaccinations.
ImmunityBio's Cancer Moonshot program (QUILT clinical trials) launched in January 2016.
ANKTIVA's commercial launch in 2024 initiates the next era of immunotherapy beyond checkpoint inhibitors based on cytokines and natural killer (NK) cells.
The company says the drug’s novel mechanism of action activates the body’s immune system of NK and killer T cells to attack BCG-resistant tumor cells and induce memory T cells, resulting in a long duration of complete response exceeding 47 months.
ANKTIVA is a first-in-class IL-15 agonist IgG1 fusion complex consisting of an IL-15 mutant (IL-15N72D) fused with an IL-15 receptor alpha, which binds with high affinity to IL-15 receptors on NK, CD4+, and CD8+ T cells.
This fusion complex of ANKTIVA mimics the natural biological properties of the membrane-bound IL-15 receptor alpha, delivering IL-15 by dendritic cells and driving the activation and proliferation of NK cells with the generation of memory killer T cells that have retained immune memory against these tumor clones.
The proliferation of the trifecta of these immune-killing cells and the activation of trained immune memory results in immunogenic cell death, inducing a state of equilibrium with durable, complete responses. ANKTIVA has improved pharmacokinetic properties, longer persistence in lymphoid tissues, and enhanced anti-tumor activity compared to native, non-complexed IL-15 in-vivo.
In the U.S., the American Cancer Society (ACS) estimates there will be 83,190 new bladder cancer cases in 2024. The annual projected cost of treatment is over $2 billion. And the average treatment cost per patient ranges from $19,521 to $169,533 (metastatic disease).
In 2024, Anktiva is priced at $35,800 per dose, separate from the cost of Merck TICE® BCG vaccines.
Furthermore, the ACS projects over 16,000 deaths from bladder cancer this year.
More information for patients and healthcare professionals is available on Anktiva.com.
Our Trust Standards: Medical Advisory Committee