"Molecular Clock" Indicates Chikungunya Arrived in Brazil during 2012

Scientists at the Center for Infection and Immunity (CII) at the Columbia Mailman School of Public Health reported that the Chikungunya virus may have arrived in Brazil from Central Africa at least one year earlier than previously assumed.
This study is important news since Brazil continues to report Chikungunya cases during 2019.
And, there is not an approved preventive vaccine for Chikungunya available today.
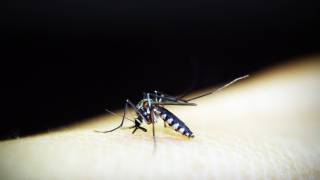
Chikungunya is a mosquito-borne viral disease and is an RNA virus that belongs to the alphavirus genus of the family Togaviridae.
This new analysis was determined by an innovative genetic test, the QuantiTect/QuantiNova Prove RT-PCR Kit.
This analysis of 14 genomic sequences showed a strong correlation between genetic divergence and the date at which the sample was taken.
This allowed the researchers to identify a "molecular clock", based on the pace of mutations between samples.
The timing suggested the virus could have circulated as early as 2012 and was likely imported from Central Africa. Chikungunya virus was first reported by Brazil’s public health surveillance systems in 2014.
"With heightened concerns around Zika, more Brazilians were tested for the infection," says Nischay Mishra, Ph.D., the study's leader at CII.
"At the same time, many of those tested were actually infected with dengue or chikungunya, which present with similar symptoms."
"This study demonstrates the value of sensitive diagnostic technologies that can differentiate between infectious diseases," says first author Thiago Moreno L. Souza, a professor of biochemistry at Fundação Oswaldo Cruz in Rio de Janeiro, Brazil.
The test identified 40 samples positive for Chikungunya virus and negative for Dengue virus and Zika virus.
Chikungunya distinctive dermatologic manifestations include facial melanosis and bullous eruption.
Researchers then retested these samples using the CII-ArboViroPlex, a multiplex test developed by CII that can simultaneously scan for the presence of Zika virus, all serotypes of dengue virus, chikungunya virus, and West Nile virus.
The CII test confirmed the earlier testing, but performed with greater sensitivity, suggesting it could identify the virus when other tests could not.
Fourteen of these samples representing dates across the 15-month collection period were further analyzed using VirCapSeq-VERT, a method developed at CII for viral diagnosis, surveillance, and discovery.
VirCapSeq-VERT enabled recovery of near-complete viral genomic sequences and identification of the viruses as representatives of the East-Central-South-African genotype of chikungunya virus.
VirCapSeq-VERT, a streamlined version of unbiased sequencing, references a library of sequences selected from among approximately 2 million genetic pieces, representing all viral taxa known to infect vertebrates.
Recently, a separate study published by the Centers for Disease Control and Prevention reported reliable diagnostic testing for acute Chikungunya infections by IgM detection from 5 dpo onward.
This finding might enable waiving labor-intensive and costly molecular protocols in many patients, minimizing costs for public health systems and cohort studies investigating arbovirus pathogenesis.
However, the reliability of Chikungunya serologic diagnostic tests must be reevaluated for co-circulating genotypes, and for the antigenically related Mayaro virus, if it emerges in Latin America.
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES); National Council for Scientific and Technological Development (CNPq); Ministry of Science, Technology, Information and Communications; Research Foundation of the State of Rio de Janeiro and Oswaldo Cruz Foundation; the National Institutes of Health (AI109761), the German Federal Ministry of Education and Research, and the People Programme (Marie Curie Actions) of the European Union's Seventh Framework Programme.
No conflicts of interest were disclosed.
Recently, a January 2019 press release said ‘a single-shot vaccination of the Chikungunya vaccine candidate VLA1553 showed excellent immunogenicity and acceptable safety profiles.’
Our Trust Standards: Medical Advisory Committee