Rabies Vaccines
Rabies Vaccines 2024
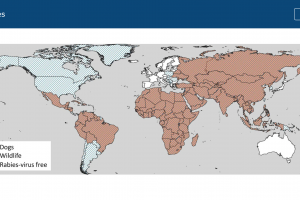
Rabies is a vaccine-preventable viral disease found in more than 150 countries and territories, according to the World Health Organization (WHO). Two types of vaccines protect people against rabies: nerve tissue and cell culture vaccines. Cell culture vaccines, which are more affordable and require less vaccine, have been developed recently. Unlike conventional, inactivated rabies vaccines, live-attenuated viruses are genetically modified viruses that can replicate in a vaccinated person without causing adverse effects while eliciting robust and effective immune responses against viral infection. According to the WHO, intradermal immunization using cell-culture-based rabies vaccines is an acceptable alternative to standard intramuscular administration. In the United Kingdom, rabies vaccines must be inactivated or recombinant and approved in the country of use.
Rabies Vaccines Approved
As of 2024, WHO pre-qualified human rabies vaccines include RABIVAX-S by Serum Institute of India Pvt. Ltd., VaxiRab N by Zydus Lifesciences Limited, and VERORAB by Sanofi Pasteur.
Chirorab®, previously known as Rabipur, is an inactivated rabies virus of Flury LEP. Chiron Behring Vaccines is re-launching Rabipur, a purified chick embryo cell vaccine. It will continue manufacturing at its WHO pre-qualified facility in Ankleshwar, Gujarat, India.
RabAvert is a vaccine that contains an inactivated rabies antigen. Bavarian Nordic's RabAvert vaccine is indicated for preexposure vaccination, primary and booster doses, and postexposure prophylaxis against rabies in all age groups.
Rabipur® is an inactivated rabies vaccine produced by Valneva SE, which has more than 35 years of worldwide clinical experience.
Rabivax-S is a lyophilized vaccine manufactured by Serum Institute of India Pvt. It contains inactivated purified rabies antigen and was developed on Vero ATCC CCL81 cells using the Pitman Moore strain. The inactivated vaccine is freeze-dried until ready for immunization.
Rabies Vaccine Candidates 2024
Rabies vaccine candidates are seeking participants for various clinical trials in 2024.
YS Biopharma Co., Ltd. PIKA Rabies Vaccine utilizes the company's proprietary PIKA adjuvant technology, designed to produce a more robust immune response in an accelerated timespan compared to existing rabies vaccines. On April 9, 2024, YS Bio announced positive interim results from the ongoing Phase 3 clinical trial, indicating that the PIKA Rabies Vaccine has successfully met the primary endpoints and has the potential to achieve best-in-class accelerated protection and meet the WHO's goal of a one-week rabies vaccine regimen to replace the conventional three- or four-week regimens. On June 1, 2023, the Food and Drug Administration of the Philippines granted Phase 3 clinical trial approval, and on May 16, 2023, Pakistan issued study approval.
Replicate Bioscience announced on September 12, 2023, the dosing of the first participant in a Phase 1 trial of its RBI-4000 vaccine candidate for rabies prevention. The Trial marks the first time a human has been dosed with Replicate's next-generation srRNA technology, which will be a benchmark for utility in this indication.
Rabies Vaccine Preexposure Prophylaxis (PrEP)
The WHO recommends preexposure prophylaxis (PrEP) for persons at high exposure risk. The WHO position paper on rabies vaccines recommends a one-week, 2-site intradermal PEP schedule with 0.1mL of vaccine injected on days 0, 3, and 7. The U.S. Centers for Disease Control and Prevention (CDC) updated its recommendations for rabies PrEP for humans, replacing the three-dose vaccination schedule with a two-dose program intended to protect people for at least three years. On May 6, 2022, the U.S. CDC published Use of a Modified Preexposure Prophylaxis Vaccination Schedule to Prevent Human Rabies: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022.
Rabies Vaccine Breakthrough Infections
A case report by Holzbauer et al. in the March 2023 issue of Clinical Infectious Diseases is not a situation of inappropriate prophylaxis; rather, it represents a textbook response to accurate exposure to rabies. An 84-year-old awoke to a bat biting his finger. The man washed his finger with soap and water, as recommended. Post-exposure prophylaxis was initiated three days after exposure, and he received a complete four doses of vaccine on schedule and more than the recommended 20 IU/kg of human rabies immunoglobulin (actual dose reportedly 30.9 IU/kg according to potency testing of the immunoglobulin used) partially injected into the bite site with the remaining amount given intramuscularly. Yet, five months later, he developed clinical rabies and succumbed to it.
Importation of Dogs Without Rabies
In the U.S., stray dog control programs were initiated in the 1940s, and routine rabies vaccination of owned dogs eliminated the canine rabies virus variant from circulation by 2008. The American Animal Hospital Association Canine Vaccination Guidelines were updated in 2022. As of August 2023, a valid CDC Rabies Vaccination and Microchip Record are needed to obtain a permit or make a reservation. The CDC does not accept foreign-issued pet passports or other certificates for foreign rabies vaccinations. Dogs vaccinated against rabies in the U.S. by a US-licensed veterinarian may re-enter the country from a high-risk country without a CDC Dog Import Permit if the dog has a current, valid US-issued rabies vaccination certificate and ISO-compatible microchip. The CDC temporarily suspended dog importation from high-risk dog rabies countries until July 31, 2024.
Rabies Vaccines Raccons
The U.S. Department of Agriculture's Animal and Plant Health Inspection Service announced on August 4, 2023, that it will begin its annual distribution of RABORAL V-RG®, an oral rabies vaccine bait, in select areas in the eastern United States to prevent the spread of raccoon rabies. Raccoons, foxes (red and gray), skunks, and bats are considered primary carriers of the rabies virus in the U.S.
Rabies Vaccine News
April 9, 2024 - Dr. Zenaida Mojares, Chief Medical Officer of YS Biopharma, commented, "The interim results of the pivotal Phase 3 Trial provide compelling evidence of the robust immunogenicity and favorable safety profile of the PIKA Rabies Vaccine."
January 9, 2024 - Texas State University - TWO RABIES RESEARCHERS EXPLAIN HOW TO PROTECT YOURSELF.
September 7, 2023 - Dog vaccination hesitancy was identified as a concern for rabies.
August 4, 2023 - The U.S. Department of Agriculture's Animal and Plant Health Inspection Service launched its annual distribution of RABORAL V-RG®, an oral rabies vaccine bait, in select areas in the eastern U.S. to prevent the spread of raccoon rabies.
June 1, 2023: The local health department announced 116 Queenslanders had potentially been exposed to the deadly rabies virus in 2023.
March 29, 2023 - Clinical Infectious Diseases reported an 84-year-old male died from rabies six months after being bitten by a rabid bat despite receiving timely rabies PEP. Clinicians should consider measuring rabies-neutralizing antibody titers after completion of PEP if there is any suspicion of immunocompromise.
January 23, 2023 - Bharat Biotech initiated a voluntary, limited recall for a batch of its anti-rabies vaccine, Chirorab®.
January 12, 2023 - Jefferson County Public Health (Colorado) encourages residents to take precautions to prevent exposure and minimize harm from rabies.
January 10, 2023 - The Texas Department of State Health Services Zoonosis Control Branch launched the 2023 Oral Rabies Vaccination Program's aerial distribution of oral rabies vaccine baits for wildlife.
December 14, 2022 - Everest Medicines announced it had achieved the preclinical proof-of-concept milestone for its mRNA rabies vaccine program, intended for rabies post-exposure prophylactic.
May 19, 2022 - New research published in Nature Communications shows that eliminating human dog-mediated rabies by combining mass dog vaccination, human education, and intensified rabies surveillance is feasible and cost-effective – at the state level.
May 5, 2022 - The U.S. CDC issued a Level 1 watch, Practice Usual Precautions, regarding the rabies outbreak in Haiti.
April 9, 2022: NPR reported that D.C. Health, the District of Columbia's health agency, confirmed that the fox tested positive for the rabies virus.
March 10, 2022 - Garrett County Health Department, located near Oakland, CA, reported the first case of laboratory-confirmed rabies for 2022, involving a gray fox attacking a person.
January 17, 2022 - Rabies was confirmed in a four-year-old child from South Africa from Eastern Cape Province. In addition, a case was confirmed as a patient who contracted the disease in Lusaka, Zambia but was hospitalized and died in Johannesburg. In 2021, a total of 19 confirmed human rabies were reported from the Eastern Cape (n=9), KwaZulu-Natal (n=6), and Limpopo (n=4) provinces.
January 7, 2022 - The U.S. CDC published Notes from the Field: Three Human Rabies Deaths Attributed to Bat Exposures — United States, August 2021.
According to a report published by Allied Market Research, the global rabies vaccine market was valued at $1,207 million in 2022 and is projected to reach $1,903 million by 2032, registering a CAGR of 4.7%. Globally, rabies causes an estimated cost of $ 8.6 billion annually, and almost 60,000 people die annually.