Pentavalent Meningitis Vaccine Reduces Africa's Meningitis Belt

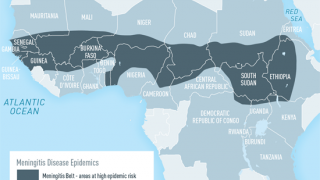
Meningitis is one of the leading causes of mortality and morbidity, especially in the meningitis belt of sub-Saharan Africa. Recurrent meningitis outbreaks across the meningitis belt have resulted in significant mortality over decades.
Sub-Saharan Africa historically experienced major epidemics of meningococcal disease every 5 to 12 years. Meningitis is a severe infection of the thin membrane surrounding the brain and spinal cord that can cause severe brain damage and is fatal in up to 80% of cases if untreated.
The U.S. CDC reports that attack rates during epidemics in Africa can reach 1,000 cases per 100,000 population.
A recent paper examined the global burden of meningitis, 2.5 million cases, exploring its prevalence and impact across different regions.
This research further analyzed the evolution of meningitis vaccination strategies. Licensed vaccines against meningococcal disease have been available for more than 40 years. Over time, there have been significant improvements in strain coverage.
These researchers highlighted the recent development and introduction of the novel pentavalent Men5CV meningococcal conjugate vaccine.
The Men5CV vaccine has emerged as a remarkable advancement in the fight against meningitis. It is safe and effective against various serogroups, including the elusive serogroup X.
The vaccine results from a 13-year development collaboration between Serum Institute of India and PATH.
The Men5CV vaccine's pre-qualification by the World Health Organization (WHO) in July 2023 and subsequent recommendation for incorporation into routine immunization programs have opened a new era with the potential for meningitis eradication.
For example, Nigeria launched vaccinations in February 2024 and now sets a benchmark for other nations in the meningitis zone, becoming the first country in the world to roll out the new Men5CV vaccines.
Funding from organizations like Gavi highlights the importance of coordinated international efforts aligned with the WHO's roadmap for meningitis elimination by 2030.
Dr. Tokunbo Oshin, Director of High Impact Countries at Gavi, the Vaccine Alliance, commented in March 2024, "Thanks to vaccines, we have eliminated large and disruptive outbreaks of meningitis A in Africa: now we have a tool to respond to other meningococcal meningitis serogroups that still cause large outbreaks resulting in long-term disability and deaths."
The U.S. CDC says meningococcal vaccines are the best way to protect against meningococcal disease, but side effects can occur.
Three types of meningococcal vaccines are used in the U.S.: Meningococcal conjugate or MenACWY vaccines, Serogroup B meningococcal or MenB vaccines, and Pentavalent or MenABCWY vaccine.
These vaccines are offered at health clinics and pharmacies in the U.S.
The CDC recommends that travelers two months old or older get vaccinated before visiting areas part of the meningitis belt during the dry season when visiting countries such as Nigeria.
Our Trust Standards: Medical Advisory Committee