Norovirus Outbreaks Return Without Approved Vaccines
Recent data published in England and the United States show high levels of patients diagnosed with norovirus, the winter vomiting bug, as the end of 2023 approaches.
The timing of norovirus outbreaks can vary considerably from one season to the next, says the U.K. Health Security Agency.
According to the U.K.'s NHS, an average of 351 people were hospitalized with diarrhea and vomiting symptoms every day at the end of November compared to 126 in the same week in 2022.
There were also 13 children with the virus in hospital each day, compared to an average of just three for the same period in 2022.
The NHS national medical director, Professor Sir Stephen Powis, commented in a recent press release, "We all know somebody who has had some kind of nasty winter virus in the last few weeks, and today's data shows this is starting to trickle through to hospital admissions, with a much higher volume of norovirus cases compared to last year."
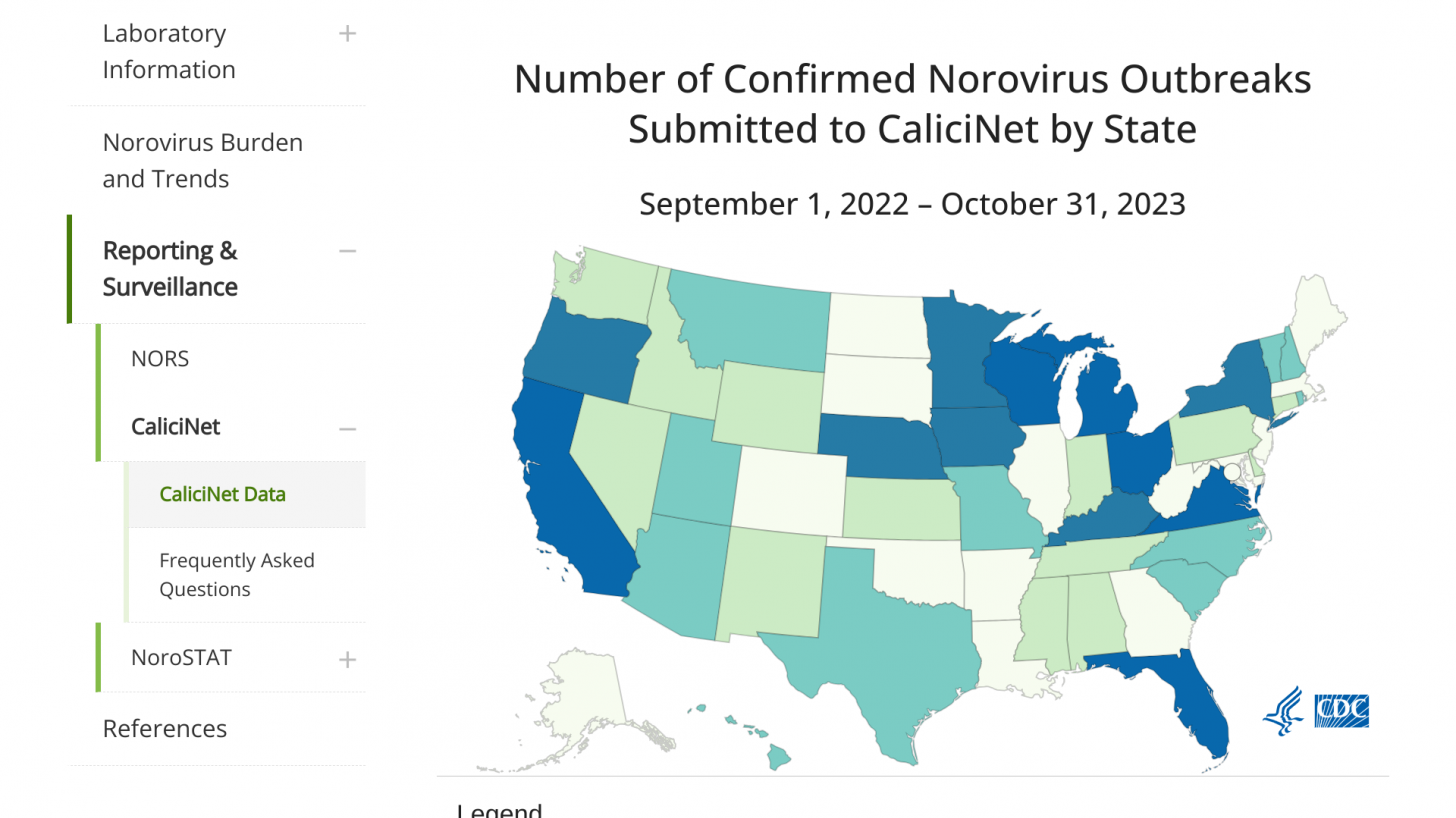
In the U.S., from August through November 13, 2023, there were 202 norovirus outbreaks reported by NoroSTAT-participating states.
California leads all states with 67 norovirus outbreaks reported this year.
During the same period last season, 134 norovirus outbreaks were reported by these states.
The most commonly detected norovirus genotype worldwide is genogroup II- genotype 4 (GII.4), which is the focus of leading vaccine candidates.
While the U.S. and U.K. have not approved a norovirus vaccine, late-stage candidates are advancing.
For example, HilleVax stated in a press release on September 20, 2023, that it intends to use the net proceeds from the recent $100 million offering to fund the clinical development of HIL-214.
".... we are adjusting our guidance to read out topline clinical data on all subjects in our NEST-IN1 phase 2/3 clinical trial to mid-2024," said Rob Hershberg, MD, PhD, Chairman and Chief Executive Officer of HilleVax.
HIL-214 is an investigational virus-like particle bivalent vaccine candidate for preventing moderate-to-severe acute gastroenteritis caused by norovirus in infants. The HIL-214 vaccine includes antigens from genotypes GI.1 and GII.4.
Our Trust Standards: Medical Advisory Committee
























