Microarray Skin Patch Successfully Delivers Measles Vaccines
The journal Vaccines recently reported that microarray patches (MAPs) have the potential to be a safer, more acceptable, easier-to-use, and more cost-effective means for the administration of vaccines than injection by needle and syringe.
Published on November 17, 2023, a phase 1 clinical trial found a single dose of measles and rubella (MR) vaccine delivered to adults by the Vaxxas high-density microarray patch (HD-MAP) provided antibody responses comparable to the same vaccine delivered by needle and syringe.
Higher antibody responses in study participants with the lowest levels of pre-existing antibodies to MR suggest HD-MAPs could be a valuable means for delivering MR-vaccine to hard-to-reach populations and support further development. wrote these researchers.
The study, completed by the University of the Sunshine Coast in Queensland, Australia, involved the delivery of an MR vaccine using the Vaxxas HD-MAP technology.
The trial involved 60 adult participants, 30 of whom received the HD-MAP-delivered vaccine at different doses; 15 received a comparative vaccine delivered by needle and syringe; and 15 received an HD-MAP-delivered placebo product.
Study participants receiving the MR vaccine delivered by HD-MAP showed a significant increase in neutralizing antibodies compared to placebo and a comparable response to the same comparator vaccine delivered with needle and syringe.
There was also a strong correlation between starting levels of neutralizing antibodies and the impact of the HD-MAP-delivered vaccine, with participants having the lowest levels of pre-existing antibodies to MR showing the strongest response after vaccination.
“We are incredibly pleased with the results of this groundbreaking clinical trial,” commented David Hoey, Vaxxas Chief Executive Officer, in a press release on November 27, 2023.
“Measles and rubella remain significant health concerns in many parts of the world, and we look forward to moving this product forward to later-stage clinical trials.”
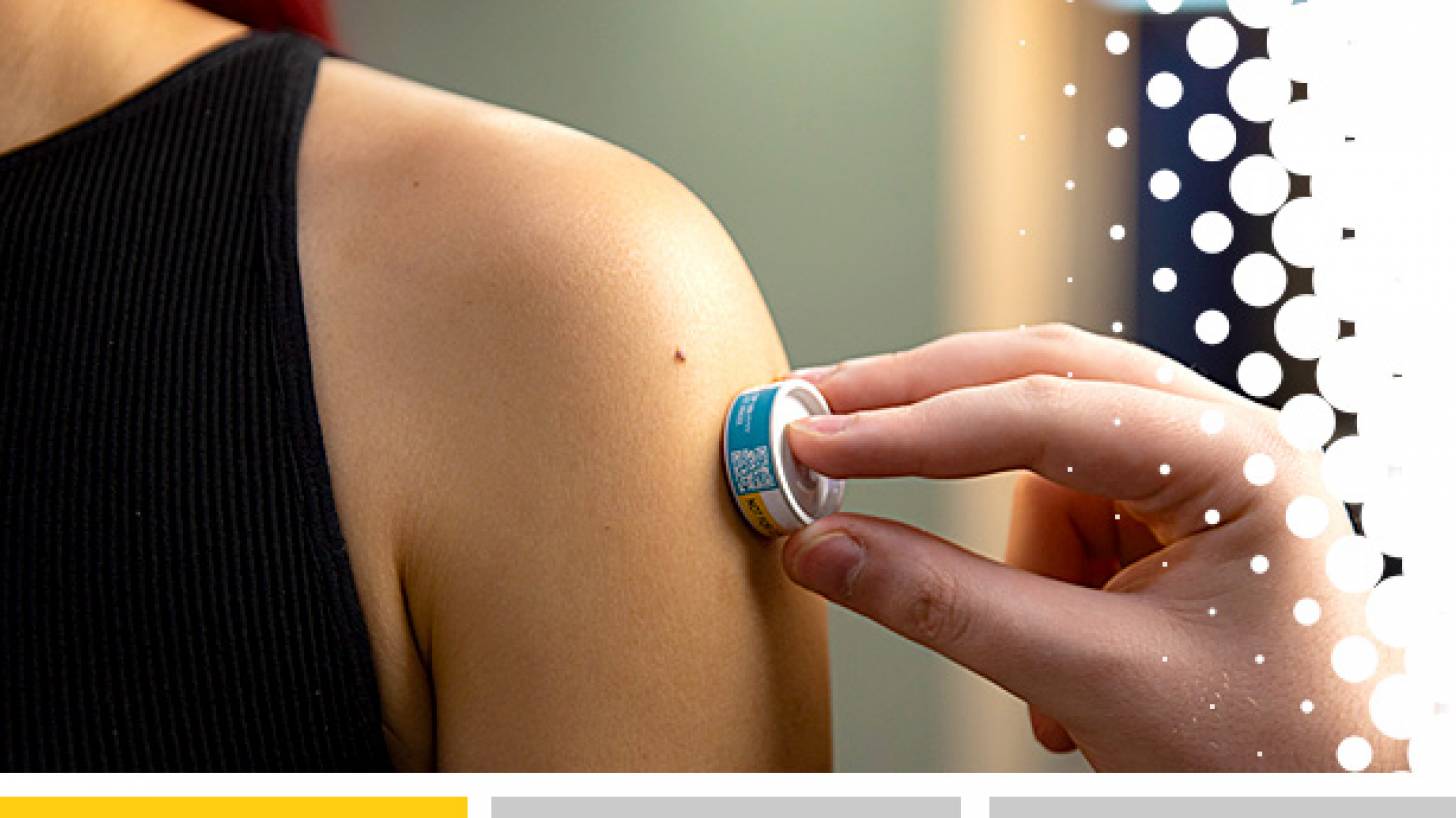
The Vaxxas HD-MAP uses a high-density array of microprojections – invisible to the human eye – applied to the skin as a patch in a small applicator device.
When applied to the skin, the patch rapidly delivers the vaccine to the abundant immune cells that are naturally present immediately below the skin surface. This approach has been shown to enhance immune responses to vaccines.
The HD-MAP technology also offers the potential to eliminate or minimize the need for vaccine refrigeration during storage and transportation – easing the resource and logistics burden of maintaining the refrigerated “cold chain.”
These researchers wrote that ease of use of the HD-MAP could enable simplified administration, potentially encompassing self-administration.
Based on this data, Vaxxas is progressing toward a Phase I/II clinical trial in Africa. This planned Phase I/II, an additional Bill & Melinda Gates Foundation grant, will partly support the MR clinical program.
Our Trust Standards: Medical Advisory Committee