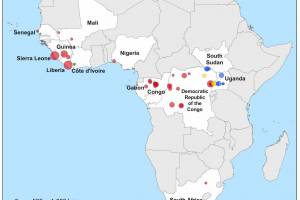
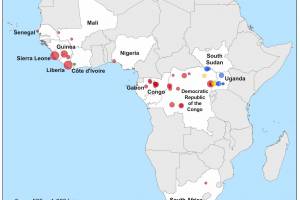
Ebola Outbreaks
Ebola Outbreaks 2025
The initial Zaire Ebolavirus disease (EBOV) case was confirmed in 1976 in a village near the Ebola River. A study published on August 18, 2023, says the origins of Ebola remain enigmatic. Recent data suggest that some EBOV outbreaks may originate from human-to-human transmission of prior Ebola outbreak strains instead of spillover. Orthoebolaviruses are a group of four viruses that cause Ebola disease. According to the World Health Organization (WHO), Ebolavirus outbreaks frequently occur in Africa. As of March 2025, more than 30 EBOV outbreaks have been reported. The WHO posted a Chronology of EBOV outbreaks.
Zaire Ebolavirus Outbreaks
Africa endured Zaire Ebolavirus outbreaks in 2014, 2016, 2018, and 2022. Over 29,000 people were infected, and more than 11,000 died. The African countries most afflicted were Sierra Leone and Liberia. Since then, the Democratic Republic of Congo (DRC) Ministry of Health has announced various outbreaks of EVD. During the 2018–2020 EVD outbreak in the eastern part of the DRC, among those offered vaccination, self-reported uptake of the Ebola vaccine was 99% (95% confidence interval (CI) [98.5–99.4]). As of August 2023, Original Research indicates that EVD surveillance systems in Liberia may fail to detect a new outbreak promptly. Specific improvements are required, and regular evaluations are recommended.
Sudan Ebolavirus Outbreak
The Uganda Ministry of Health declared its eighth Sudan Ebolavirus (SVD) outbreak on January 2025. This SVD outbreak has recorded 12 cases as of March 8, 2025. The U.S. CDC reissued a Travel Health Advisory Level 2, Practice Enhanced Precautions notice on March 12, 2025, regarding the current SVD outbreak. The CDC issued Health Alert Network Health Advisory CDCHAN-00477 on October 6, 2022, and CDCHAN-00480 on November 7, 2022. Since October 2022, all U.S.-bound passengers from Uganda have been routed to designated airports for enhanced Ebola screening. The U.S. government conducted similar airport exit and entry screening for Zaire Ebolavirus in 2014. Seperately, traveler screening at Ugandan entry points remains active in 2025, with 25,364 travelers screened for SVD as of March 2, 2025.
On May 8, 2023, North Kivu, DRC, confirmed a positive case of SUDV. This revelation was made by the communicator of the provincial health division of Butembo, Dps-Butembo, and Damulo Luhavo, during a press briefing. "Regarding Ebola virus disease surveillance, we received a sample that turned out to be positive from the Kyondo health zone and in the Butembo site. We have received four samples, and among the four, one is positive," said Damulo Luhavo.
The UKHSA issued a public health message on November 14, 2022, regarding the SVD outbreak in Uganda. All workers returning to the U.K. from areas affected by SUDV should be risk-assessed by the UKHSA. On November 1, 2022, the WHO advised against any travel and/or trade restrictions to Uganda based on available information for the current SUDV outbreak.
Ebola in the United States
The U.S. CDC updated its Ebola Overbreak History on August 31, 2023. The CDC says that 11 people were treated for EVD in the U.S. during Africa's 2014-2016 epidemic. On September 30, 2014, the CDC confirmed the first travel-associated case of Zaire EVD was diagnosed in the U.S. in a traveler from West Africa to Dallas, Texas. The patient (the index case) died on October 8, 2014. Two healthcare workers who cared for him tested positive for EVD, and both recovered. On October 23, 2014, a medical aid worker who had volunteered in Guinea was hospitalized in New York City, NY, and was diagnosed with EVD. The aid worker recovered, and seven others were cared for in West Africa. Six of these EVD patients recovered; one died, reported the CDC.
Ebola Vaccines
Ebola vaccine information is posted at this Vax-Before-Travel link. In 2025, Ebola vaccines will not be commercially available in the U.S.
Ebola Therapy
The U.S. Food and Drug Administration authorized Ebanga for intravenous injections on December 21, 2020.
Ebola Prevention and Control Guidelines
In August 2024, the WHO published updated infection prevention and control research priorities in healthcare settings. Key recommendations are summarized in The BMJ. On December 18, 2023, Texas Biomed announced findings published in the Journal of Infectious Diseases (Sept. 2023) indicating that the Ebola virus creates and uses intercellular tunnels to move from cell to cell and evade treatments. "Our findings suggest that the virus can create its hiding place, hide, and then move to new cells and replicate," says Olena Shtanko, Ph.D., an Assistant Professor at Texas Biomed and senior author.
Ebolavirus Diagnostic Tests
A novel patch-based ebolavirus diagnostic test was announced on August 18, 2023.
Ebolavirus Disease
The Ebolavirus family Filoviridae includes three genera: Cuevavirus, Marburgvirus, and Ebolavirus. Within the genus Ebolavirus, six species have been identified: Zaire, Bundibugyo, Sudan, Taï Forest, Reston, and Bombali. Ebola viruses (EBOV) assemble into filamentous virions whose shape and stability are determined by the matrix viral protein 40 (VP40). The pH-driven structural remodeling of the VP40 matrix acts as a molecular switch coupling viral matrix uncoating to membrane fusion during EBOV entry. According to the WHO, EVD is transmitted to people from wild animals and spread through human-to-human transmission, with case fatality rates varying from 25% to 90%. Infection with the Ebola virus to symptom onset, including fever, fatigue, muscle pain, headache, and sore throat, can range from 2 to 21 days.