Chikungunya Outbreak Becomes an India Public Health Emergency
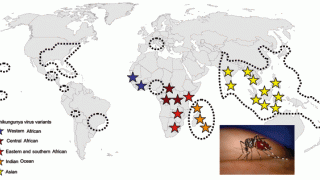
While most of the media attention has been focused on the continued detention of Chikungunya virus disease (CHIKVD) cases in the Region of the Americas, this mosquito-borne disease has also affected Asian countries.
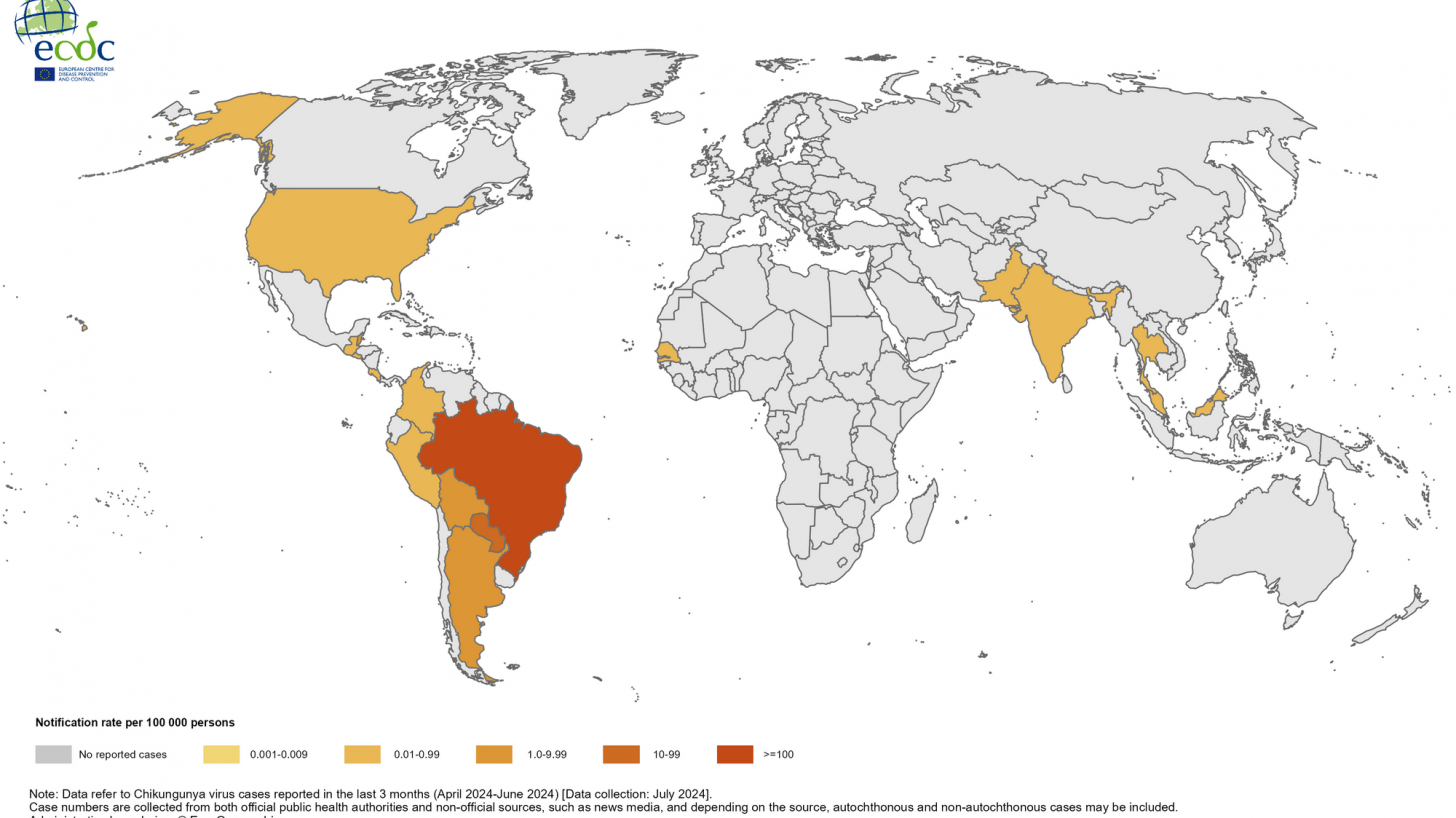
As of early August 2024, the European Centre for Disease Prevention and Control reported CHIKVD outbreaks from Pakistan (1,141), the Maldives (389), Thailand (256), India (243), Timor Leste (195), and Malaysia (34).
The most alarming development has been the sudden increase in cases in India. Chikungunya was initially reported in India in 1963 but re-emerged in 2005. Currently, every part of the country has become endemic for CHIKVD.
In August alone, 167 cases were reported.
In response to a test positivity rate exceeding 20%, the Nagpur Municipal Corporation's health department declared a public health emergency after confirming 35 CHIKVD cases in early August.
This declaration is significant because Nagpur, India, has a population of over 3 million residents.
Moreover, according to a recent study, CHIKVD's health risk continues for months.
Chikungunya is an arbovirus disease whose symptoms develop after 2–6 days of incubation. These include high fever and severe arthralgia.
The Lancet Infectious Diseases published results from a study published in February 2024 investigating the risk of death in people infected with Chikungunya two years after the first symptoms of the disease.
This study concluded Chikungunya infection was associated with an increased risk of death from various causes for up to 84 days after symptom onset.
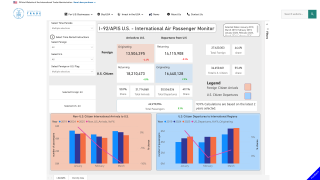
According to the U.S. CDC, Chikungunya infections are a measurable risk for international travelers.
With India's foreign tourism rebounding, reaching about 10 million annual visitors again, and the significant CHIKVD outbreak in the Region of the Americas, having access to an effective travel vaccine is essential.
As of August 22, 2024, 57 travel-related CHIKV cases were confirmed in the U.S. this year.
To help prevent infections, the U.S. FDA recently announced good news when it approved Valneva SE's IXCHIQ®, the first Chikungunya vaccine. It is a monovalent, single-dose, live-attenuated Chikungunya vaccine recommended for adult travelers to endemic areas.
Our Trust Standards: Medical Advisory Committee