Another Herpes Vaccine Candidate Flops

Given the unmet medical need and burden associated with genital herpes, the approval of an innovative vaccine is much anticipated. Unfortunately, developing protective herpes simplex virus (HSV) vaccines has been challenging for decades.
However, the wait will continue according to GSK plc's recent announcement.
On September 12, 2024, GSK confirmed that it had completed the primary objective data analysis from the phase II part of the TH HSV REC-003 clinical trial. This trial is a combined phase I/II proof-of-concept study to assess the potential clinical efficacy of GSK3943104, an early-stage therapeutic herpes simplex virus (HSV) vaccine candidate.
Results from this study show that GSK3943104 did not meet the study's primary efficacy objective.
Therefore, this vaccine candidate will not progress to phase III studies. GSK wrote that it is working with study investigators to inform the participants.
The good news is that GSK's TH HSV REC-003 study will continue for routine safety monitoring and to generate follow-up data that could offer valuable insights into recurrent genital herpes. GSK said it intends to evaluate the totality of all these data and other studies to progress future research and development of its HSV program.
GSK previously terminated a SAM-based herpes vaccine approach, GSK4108771A, in 2021.
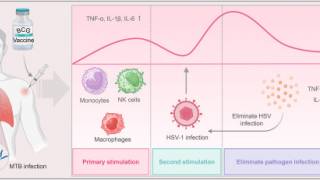
Genital herpes is a common infection caused by HSV-1 or HSV-2, which has led to millions of genital ulcers or neonatal herpes infections. Furthermore, about 30% of pregnant women in the United States have genital infection with HSV.
As of September 2024, current herpes vaccine candidates are based on DNA, modified mRNA, protein subunits, killed virus, and attenuated live virus vaccine technologies. Two mRNA vaccine candidates, mRNA-1608 and BNT163, are progressing through clinical trials.
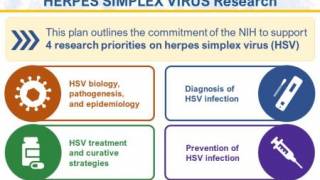
The U.S. National Instuties of Health and global partners have launched STI Watch, a digital portal containing updated information on vaccine development status. As of September 2024, no country has approved a herpes vaccine.
Our Trust Standards: Medical Advisory Committee