Bladder Cancer Immunotherapy Revenues Increase 129%

ImmunityBio, a vertically integrated biotechnology company developing next-generation therapies and vaccines that enhance the natural immune system to combat cancers and infectious diseases, recently reported a significant increase in revenue.
On April 15, 2025, ImmunityBio announced that it earned net product revenue of approximately $16.5 million during the three months ended March 31, 2025, representing a 129% increase over the net revenue earned during the fourth quarter of 2024.
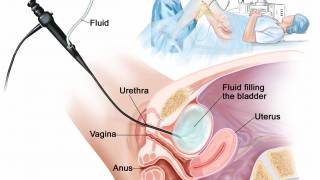
With the issuance of the permanent J-code (J9028) in January 2025, ImmunityBio has experienced increased sales momentum for ANKTIVA®, the first U.S. FDA-approved immunotherapy for non-muscle-invasive bladder cancer that activates natural killer cells, T cells, and memory T cells to elicit a long-duration response.
The Company's range of immunotherapy and cell therapy platforms, both individually and in combination, act to drive and sustain an immune response, to create durable and safe protection against disease.
ANKTIVA's triangle offense against cancer includes natural killer cells, T cells, and memory T cells.
ANKTIVA (N-803) (nogapendekin alfa inbakicept-pmln) is a first-in-class interleukin-15 agonist IgG1 fusion complex consisting of an IL-15 mutant (IL-15N72D) fused with an IL-15 receptor alpha, which binds with high affinity to IL-15 receptors on NK, CD4+, and CD8+ T cells.
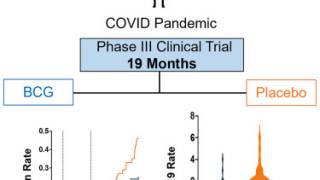
As of April 2025, the ANKTIVA plus BCG vaccine became available for treating bladder cancer patients at various clinical sites in the United States.
Our Trust Standards: Medical Advisory Committee