Brazil Authorizes Chikungunya Vaccine

With over 1 million chikungunya cases reported over the last decade, the residents of the Federative Republic of Brazil have gained access to an effective vaccine that prevents this mosquito-transmitted disease.
Valneva SE today announced that the Brazilian Health Regulatory Agency has granted marketing authorization to its single-dose vaccine IXCHIQ®.
This innovative and safe vaccine prevents disease caused by the chikungunya virus in individuals 18 and older.
Additionally, Valneva and Instituto Butantan confirmed they are working together to ensure fast access to chikungunya vaccines for the Brazilian market and other countries in the region as quickly as possible.
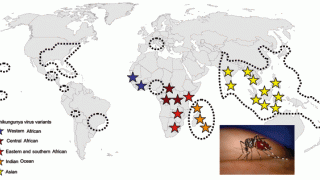
The Region of the Americas reported various outbreaks. Between 2013 and 2023, there were more than 3.7 million chikungunya cases in the Americas. Brazil has reported over 1 million cases in the past few years.
Juan Carlos Jaramillo, M.D., Chief Medical Officer of Valneva, commented in a press release on April 14, 2025, "The ongoing outbreak in Brazil underscores the fact that containing chikungunya is an international public health priority."
Part of Valneva's endemic country strategy, this endeavor is supported by the Coalition for Epidemic Preparedness Innovations, with co-funding from the European Union and Instituto Butantan to support broader access to a chikungunya vaccine in low- and middle-income countries.
Dr. Esper Kallás, Director of Instituto Butantan, added, "The approval of the chikungunya vaccine is a great victory for Brazil, where over 150,000 people suffer from the disease yearly. It is an honor for Butantan to be able to contribute to ensuring that this vaccine reaches the population that needs it the most."
IXCHIQ® is the world's first licensed chikungunya vaccine. It is approved to prevent disease caused by the chikungunya virus in people aged 12 years and older in the EU and 18 years and older in the United States, Canada, and the United Kingdom. Label extension applications to adolescents were submitted in the U.S., Canada, and the U.K.
In the U.S., IXCHIQ is commercially offered at travel clinics and pharmacies.
Our Trust Standards: Medical Advisory Committee