$2.6 Million Funds Another MERS Vaccine Candidate

While this respiratory disease is generally related to camel interactions in the Kingdom of Saudi Arabia, clinical efforts to produce a Middle East Respiratory Syndrome (MERS) vaccine have been elusive.
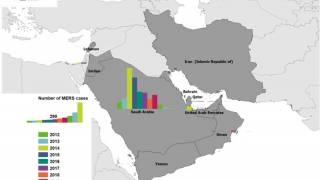
Since April 2012 and as of mid-March 2025, six World Health Organization regions have reported 2,618 cases of MERS, including 945 deaths, a significant case-fatality rate.
To address this need, CEPI announced, on March 25, 2025, a $2.6 million investment in moving a promising vaccine candidate into preclinical trials.
This new investment, developed by Newark, DE-based Uvax Bio, an early-stage vaccine technology company spun out of The Scripps Research Institute, is based on proprietary protein nanoparticle technology, 1c-SApNP®, licensed from Scripps Research.
The technology is already being tested against other infectious diseases, including HIV, where an in-human trial is ongoing.
Dr. Kent Kester, Executive Director of Vaccine R&D, CEPI, commented in a press release, “Uvax Bio’s unique vaccine could help strengthen our response to future MERS outbreaks while informing us of the vaccines being developed against other coronaviruses.”
Uvax’s novel vaccine design uses tiny protein “nanoparticles” to closely resemble or mimic the size and shape of the MERS coronavirus.
Uvax Bio has analyzed viral structures and designed the technology to present enhanced antigens —parts of the virus that trigger an immune response—in a multilayered scaffold layout. This design offers stability and allows for as many as twenty antigens to be presented at once, which could help provide strong protection by generating both antibody and T-cell immunity.
The 1c-SApNP® technology is also unique as it has been combined with a process called ‘glycan trimming.’
Here, sugar molecules—called glycans—that would generally cover the MERS virus are shortened in the nanoparticle virus-mimicking vaccine design. This could expose additional sites on the antigen surface, enhancing the immune response.
In addition to the vaccine candidate, several MERS vaccines are in clinical development addressing this zoonotic disease with an unknown source in 2025.
Our Trust Standards: Medical Advisory Committee