20,000 Frontline Workers to Receive Ebola Vaccination
A decade after the deadliest Ebola virus disease (EVD) outbreak in history, Sierra Leone will become the first country to launch a preventive Ebola vaccination campaign targeting 20,000 frontline workers in all 16 districts across the country.
A single dose of the Ebola vaccine Ervebo will be administered to health care professionals, frontline workers, and first responders such as motorbike riders/ambulance drivers, traditional healers, religious leaders, security forces, and others who are at high risk of being exposed to EVD.
"Protecting our frontline workers is vital to our National Health Security Plan, ensuring preparedness and resilience against future health threats. This is an investment in the safety of our people and a healthier Sierra Leone," said Dr Austin Demby, Minister of Health, Sierra Leone, in a press release on December 4, 2024.
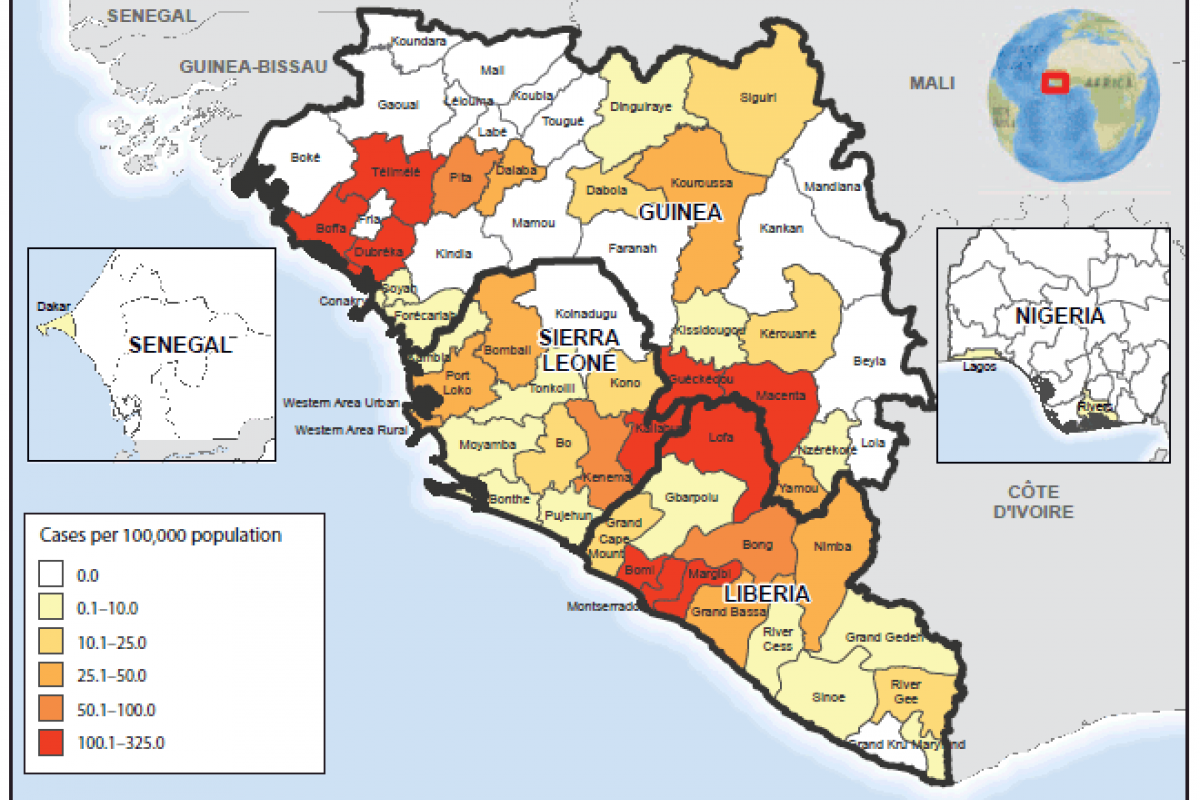
This preventive vaccination campaign comes after the deadly 2014–2016 Ebola outbreak was declared a Public Health Emergency of International Concern. That outbreak resulted in more than 11,000 deaths in 10 countries around the world.
Of these, Sierra Leone reported close to 4,000 deaths.
The first cases of EVD were detected in Sudan and the Democratic Republic of the Congo in 1976. The initial Zaire Ebolavirus outbreak was confirmed in a village near the Ebola River.
Merck Ervebo® Ebola Vaccine (rVSV-ZEBOV-GP, rVSV-ZEBOV, v920) is a live, recombinant, replication-competent Orthoebolavirus zairense vaccine that the U.S. FDA approved on December 19, 2019. Orthoebolaviruses are a group of four viruses that cause EVD.
Vaccines for the Sierra Leone campaign are sourced from the Gavi-funded global vaccine stockpile administered by the International Coordinating Group on Vaccine Provision.
Ervebo is not commercially available in the U.S.
Our Trust Standards: Medical Advisory Committee