Two Mpox Vaccines Deployed in the Congo
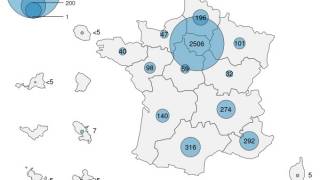
Since the start of 2024, the Democratic Republic of Congo (DRC) has reported over 20,000 mpox cases, with more than 1,000 deaths, primarily affecting children.
In June 2024, the U.S. CDC issued a Level 2 Alert reporting a mpox outbreak in 25 out of 26 DTC provinces, including urban areas.
According to media sources, authorities in the DRC have responded to this outbreak by approving the use of two new vaccines.
AfricaNews.com reported on June 28, 2024, that emergency use authorization had been issued for the Jynneos® vaccine, developed by Bavarian Nordic, and LC16, produced by KM Biologics.
LC16 is a 3rd generation, live attenuated vaccine containing live vaccinia virus (LC16m8 strain) used to prevent smallpox and mpox.
The DRC decision follows rigorous evaluation by relevant authorities and stakeholders involved in the authorization process.
JYNNEOS (MVA-BN®, IMVAMUNE®) is a two-dose vaccine based on a live, attenuated vaccinia virus, Modified Vaccinia Ankara, and has been offered in the United States since May 2022.
Our Trust Standards: Medical Advisory Committee