Multi-Country Herpes Immunotherapy Clinical Study Continues
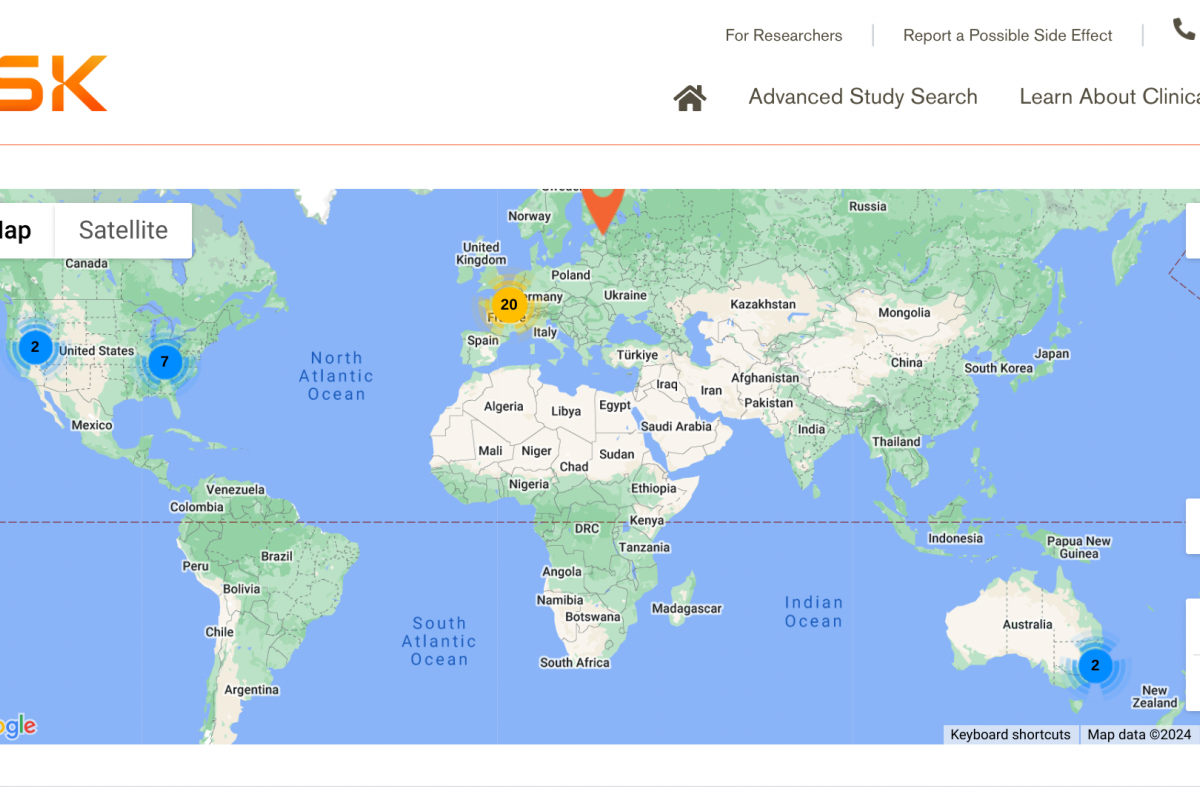
A leading global vaccine developer has announced that their investigational vaccine candidate for Herpes Simplex Virus (HSV) is undergoing a phase 1/2 clinical trial for the first time in Europe, England, and the United States.
GlaxoSmithKline plc. (GSK), known for its extensive range of vaccines for various conditions, including RSV, meningitis, and shingles, is conducting an innovative study to evaluate the safety, immune response, efficacy, and reactogenicity of HSV-targeted immunotherapy (HSVTI).
This multi-country research study is recruiting 332 healthy participants aged 18-40 years or those aged 18-60 years with recurrent genital herpes.
Known as GSK3943104A, GSK Study #215336 is forecasted to conclude in May 2026.
This HSVTI study will explore different formulations and be conducted in two parts:
Part I assesses different formulations of the HSVTI in healthy participants;
Part II assesses the two formulations of the HSVTI in participants aged 18-60 years with recurrent genital herpes.
In 2023, the World Health Organization estimated 3.7 billion people under the age of 50 have a HSV infection. Genital herpes is a sexually transmitted infection (STI) passed on through vaginal, anal, and oral sex.
There are approved vaccines to prevent STIs, such as mpox and HPV.
Currently, there's no cure or preventive vaccine for genital herpes.
However, in addition to GSK, companies such as Moderna Inc and BioNTech SE are conducting HSV vaccine research as of March 5, 2024.
Our Trust Standards: Medical Advisory Committee