Innovative Rabies Vaccine Candidate Completes Phase 3 Enrollment

YS Biopharma Co., Ltd. today announced the completion of enrollment in its Phase 3 clinical trial of the PIKA rabies vaccine. The clinical trial will include 4,500 subjects and assess the vaccine's safety, immunogenicity, and lot-to-lot consistency.
Previous Phase 1 and Phase 2 clinical trials of the PIKA Rabies Vaccine have demonstrated its safety and strong immunogenicity, with the PIKA Rabies Vaccine eliciting a detectable immune response in as quick as seven days.
Given these results, the PIKA Rabies Vaccine has the potential to achieve best-in-class accelerated protection and meet the World Health Organization (WHO) goal of a one-week rabies vaccine regimen to replace the conventional three- or four-week regimens.
The PIKA Rabies Vaccine utilizes YS Biopharma's proprietary PIKA adjuvant technology and is designed to produce a more robust immune response in a shorter time span than existing rabies vaccines.
As of October 31, 2023, there are several approved rabies vaccines available.
In a press release, Dr. Zenaida Mojares, Chief Medical Officer of YS Biopharma, commented, "We remain dedicated to leveraging our advanced PIKA adjuvant technology to enhance global health and well-being, and are thrilled to explore the near-term and long-term possibilities it offers."
According to the WHO, rabies has an almost 100% fatality rate upon emergence of clinical symptoms. Each year, it claims the lives of approximately 59,000 individuals in more than 150 countries.
Although rabies is typically lethal without treatment, the administration of post-exposure prophylaxis can effectively prevent fatalities when initiated following possible exposure.
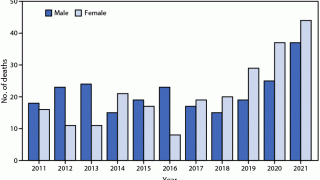
According to the U.S. CDC, human rabies cases are rare in the United States, with only one to three reported annually.
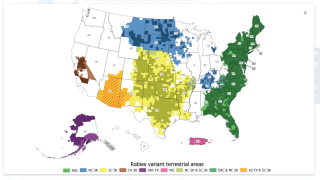
Rabies virus is transmitted through direct contact with saliva or brain/nervous system tissue from an infected animal.
In the U.S., people usually get rabies from the bite of a rabid bat, not dogs.
Rabies vaccination is just one of several travel vaccines recommended when visiting at-risk countries, such as Haiti.
Our Trust Standards: Medical Advisory Committee