Gonorrhoea Vaccine Candidate Fast Tracked in the U.S.

GSK plc today announced the U.S. Food and Drug Administration (FDA) had granted a Fast Track designation for its Neisseria gonorrhoeae investigational vaccine (NgG).
Fast Track designation is intended to facilitate the development and expedite the review of potentially important new drugs and vaccines to treat or prevent serious conditions with unmet medical needs.
As of June 27, 2023, the vaccine candidate is conducting a Phase II clinical trial and aims to demonstrate proof of concept by assessing the efficacy of the NgG vaccine in healthy adults.
Phil Dormitzer, Global Head of Vaccines R&D, GSK, commented in a related press release, "This designation recognizes the potential for a vaccine that could help protect millions of people across the world against the serious health consequences of infection with a bacterium that is considered a 'high priority' pathogen by the World Health Organisation."
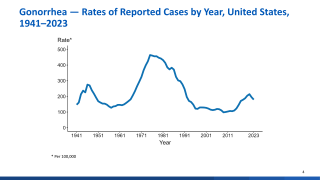
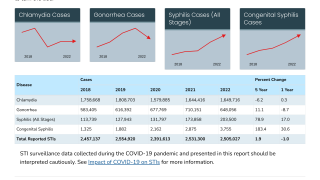
Gonorrhoea is the second most prevalent bacterial sexually transmitted infection worldwide, with an estimated 82 million new cases yearly.
In the U.S., rates of reported gonorrhea have increased by 118% from 2009 to 2021.
Furthermore, antimicrobial resistance to gonorrhea has increased over the past 80 years, rendering many classes of antibiotics used to treat the disease ineffective.
Vaccines can play a critical role in the fight against AMR by helping prevent bacterial, viral, and other infections.
Currently, no gonorrhea-specific vaccines are approved anywhere in the world, says GSK.
However, in France, the meningococcal (MenB-4C) vaccine is recommended against gonorrhea.
And Intravacc's Avacc 11® is the prophylactic intranasal gonorrhea candidate vaccine.
As of June 28, 2023, gonorrhea vaccine and treatment news have been published by Precision Vaccinations.
Our Trust Standards: Medical Advisory Committee