The U.S. FDA's Center for Biologics Evaluation and Research is conducting the 187th meeting of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) today.
This committee of vaccine experts will discuss the strain selection for Influenza Virus Vaccines for the 2025 Southern Hemisphere flu season on October 10, 2024. Each year, the VRBPAC discusses how the influenza virus is evolving and if it impacts exciting flu shot efficacy.
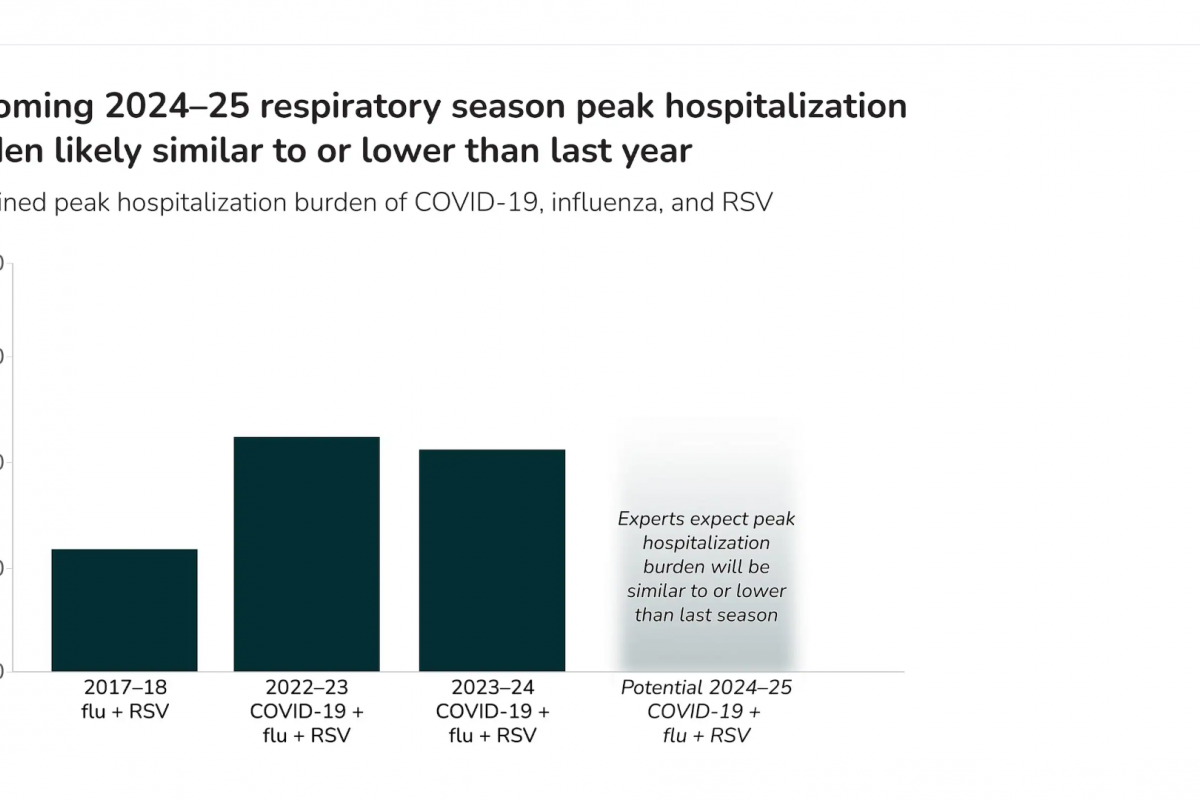
On October 3, 2024, the U.S. CDC reported that five Southern Hemisphere countries reported flu shots were about 35% effective during the last flu season.
Previously, on September 27, 2024, the World Health Organization announced its recommendations for the viral composition of influenza vaccines for the 2025 influenza season in the Southern Hemisphere.
Another presentation on the agenda is the 'Highly Pathogenic Avian Influenza A(H5Nx) Virus Surveillance and Characterization' in the U.S. and globally, and review recommendations for candidate vaccine virus development.
Over the past few months, the U.S. government has substantially invested in developing preventive vaccines for a potential avian influenza pandemic.
Instructions on listening to this VRBPAC meeting are found at this FDA link.