Testing ‘Sweet CARs’ in Cancer Patients

A Philadelphia based biotherapeutics company announced that it has dosed the first patient in its Phase 1 CART-TnMUC1-01 clinical trial with the Tn/STn glycoform of mucin 1 (TnMUC1) chimeric antigen receptor T-cell (CAR-T) therapy in patients with TnMUC1-positive advanced cancers.
Tmunity Therapeutics, Inc. said in a press release on January 15, 2020, that ‘this is a first-in-human trial with a CAR-T therapy targeting this antigen.’
The CART-TnMUC1 therapy was developed by a University of Pennsylvania (Penn) team led by Avery D. Posey, Ph.D., an assistant professor of pharmacology and a member of Penn’s Abramson Cancer Center.
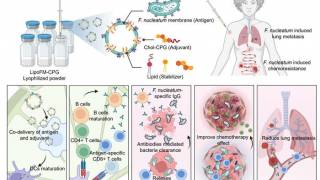
The MUC1 glycoprotein is a transmembrane epithelial mucin normally expressed on the apical surface of most simple glandular epithelial cells. In tumors that arise from these cells, an alternate form of MUC1 is frequently overexpressed on the cancer cell surface.
The alternate form, known as TnMUC1, is depolarized and aberrantly glycosylated. Tmunity’s cell therapy specifically targets the Tn (GalNAca1-O-Ser/Thr) and sialyl-Tn (STn) (NeuAca2-6-GalNAcal-O-Ser/Thr) glycoforms of MUC1 with a fusion protein recognizing a short peptide sequence with one or two Tn O-glycans on the Ser/Thr residues.
Tmunity’s CAR-T therapy also contains within its construct a novel co-stimulator region known as CD2.
“The major challenge in the field of CAR-T cells is targeting solid tumors, and we have great hope that we have potentially identified a new therapeutic approach,” Dr. Posey said.
“Our approach, testing these ‘sweet CARs,’ is to target a sugar that is uniquely expressed in tumor cells, with the aim to develop a more effective cancer therapy.”
The CART-TnMUC1-01 study is a Phase 1 trial primary objective of the trial is to establish safety and the recommended Phase 2 dose of CART-TnMUC1 that can be administered with lymphodepletion.
The trial is currently open to patients with TnMUC1-positive, treatment-resistant solid tumors, including: metastatic ovarian cancer (including cancers of the fallopian tube), pancreatic ductal adenocarcinoma, hormone receptor (HR)-negative and HER2-negative (triple-negative) breast cancer (TNBC) and non-small cell lung cancer.
Tmunity is a private clinical-stage biotherapeutics company focused on saving and improving lives by delivering the full potential of next-generation T-cell immunotherapy to patients with devastating diseases. Integrating a foundational collaboration with the University of Pennsylvania with the groundbreaking scientific, clinical, and manufacturing expertise.
For more information TMUNITY.
Our Trust Standards: Medical Advisory Committee