2nd Generation Zika Vaccine Receives $5.4 Million Dollars Funding

An Austrian company and the ZIKAVAX Consortium announced the initiation of a Phase 1 clinical trial of a 2nd generation Zika vaccine, MV-ZIKA-RSP.
This Zika vaccine candidate is based on Themis Bioscience’s proprietary measles vector platform, which includes exclusively licensed technology from Institut Pasteur.
Announced on October 1, 2019, the observer-blinded, randomized clinical trial will investigate the safety and tolerability of the novel MV-ZIKA-RSP Zika vaccine formulation in 48 healthy volunteers. The vaccine will be administered by intramuscular injection in 2-dose levels.
The secondary objectives will include optimal dose-finding, immunogenicity, and long-term safety evaluation, as well as cell-mediated immunity specific to the Zika antigen.
This is the 2nd Zika vaccine program based on Themis’ measles vector platform to enter the clinic.
The Phase 1 program and further development will be run by Themis and this first trial will evaluate the safety and immunogenicity of the candidate in healthy volunteers.
The 4-year collaborative project covers pre-clinical and clinical development and is funded under the European Union’s Horizon 2020 Research and Innovation Program (H2020 grant agreement No 732432) with an overall budget of about US $5,447,250.
A previous Themis-developed Zika program was funded through an InnovateUK grant and clinical evaluation is ongoing.
“Zika remains a global health burden with no existing vaccines, a situation exacerbated by the ongoing increase of the geographical areas exposed to the virus,” said Erich Tauber, M.D., CEO of Themis Bioscience, in a press release.
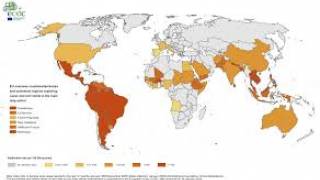
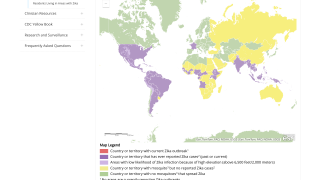
During 2019, the majority of Zika infections reported by the World Health Organization and the US Centers for Disease Control and Prevention (CDC) have been classified as ‘travel-related.’
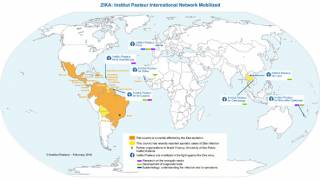
The Zika virus has recently been reported as endemic in several countries, such as the following:
- UK Travelers Avoided the Zika Virus
- Zika Arrives in Israel Again
- Travel-Related Zika Cases: September 2019
“Together with the ZIKAVAX consortium, we have applied the immune modulation potential of our measles vector approach as well as our growing clinical development expertise to provide an urgently needed Zika vaccine,” concluded Dr. Tauber.
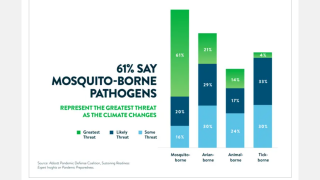
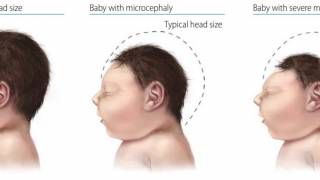
This is important news since a Zika infection during pregnancy can cause a serious birth defect, called microcephaly that is a sign of incomplete brain development, says the CDC.
To fully appreciate the health risk from the Zika virus, new research published on September 5, 2019, reported a Zika infection is also toxic to the adult brain.
Contrary to the previous belief that Zika only infects neuronal progenitor cells or neurons that are still immature in the developing brain, they found that the virus-infected and replicated in adult human tissue, producing new viral particles capable of infecting more cells.
In the USA, as of September 7, 2019, the states of Florida, Idaho, Nebraska, New Jersey, New York, and Utah reported travel-related Zika cases during 2019.
The CDC continues to alert international travelers of the risk saying ‘since there is no vaccine to prevent Zika, the best way to prevent diseases spread by mosquitoes is to protect yourself and your family from mosquito bites.’
And, avoid visiting countries with ongoing Zika virus outbreaks, such as Cuba, Mexico, and Puerto Rico.
News published by Zika News
Themis is developing immunomodulation therapies for infectious diseases and cancer. The Company will apply its platform and commercial manufacturing capabilities to diseases with high market potential both alone and for its partners. For more information, visit Themisbio.
Our Trust Standards: Medical Advisory Committee