Marburg Vaccine Candidate Now Tested in the U.S.
Since the first Marburg virus disease (MVD) outbreak in 1967, a preventive vaccine has not been available. An innovative vaccine candidate is now being tested in the United States.
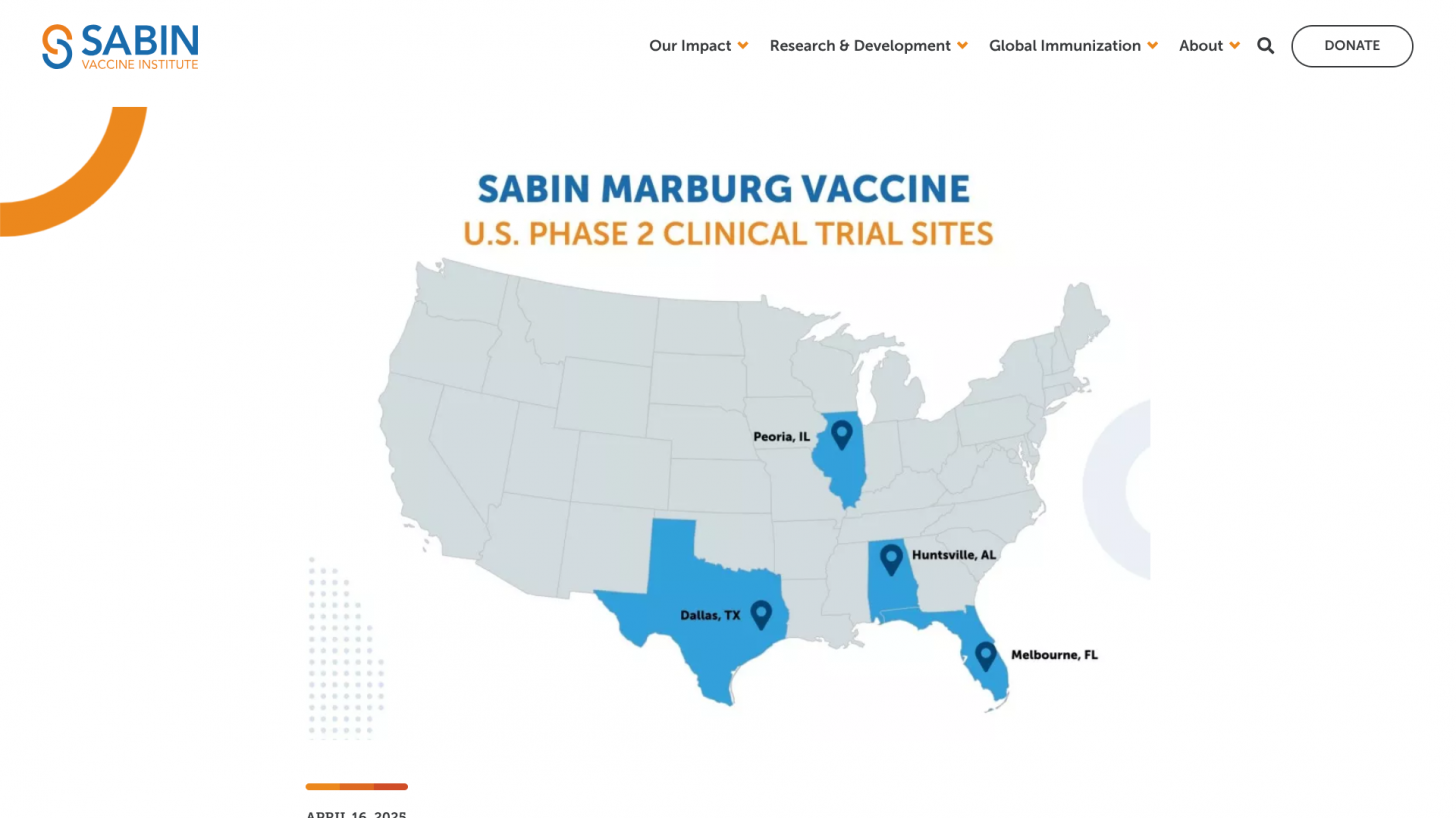
Announced on April 16, 2025, the Albert B. Sabin Vaccine Institute recently launched a multi-site Phase 2 clinical trial in the U.S. for its Marburg vaccine candidate, based on the cAd3 platform, administering doses to the first participants in Melbourne, Florida.
In addition to Melbourne, the vaccine will soon be tested at sites in Dallas, Texas; Huntsville, Alabama; and Peoria, Illinois.
The initial compaction date for this phase 2 study is April 2026.
The goal of this clinical trial is to build on ongoing Phase 2 testing in Kenya and Uganda, with initial findings from that research expected in the coming months.
Sabin's single-dose investigational Marburg vaccine was found to be promising in Phase 1 clinical and non-clinical studies, with results showing it to be safe while eliciting rapid and robust immune responses.
"Conducting clinical trials in Africa is key to evaluating the vaccine in regions where Marburg and other filoviruses are most common or endemic," notes Kelly Warfield, Sabin's President of Research & Development, in a press release.
"The U.S. trial will give us vital safety and immune response data for non-endemic populations, helping us better prepare for outbreaks and the spread of this disease."
Currently, about five other MVD vaccine candidates are conducting clinical research.
Marburg virus is a Filovirus that causes a severe and often fatal viral hemorrhagic fever, with mortality rates up to 88%.
The first outbreak of MVD occurred in 1967 in West Germany, affecting 29 people, seven of whom died. These patients had direct contact with grivets imported from Uganda.
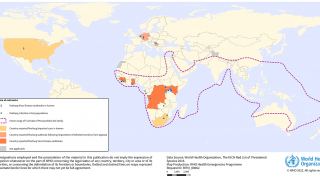
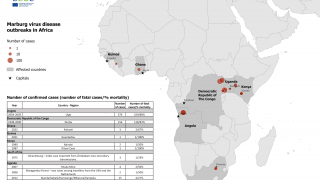
As of April 21, 2025, Angola, the Democratic Republic of the Congo, Equatorial Guinea, Cameroon, Ghana, Guinea, Kenya, Serbia, South Africa, Tanzania, Uganda, and Rwanda have confirmed cases of MVD.
The U.S. Centers for Disease Control and Prevention (CDC) previously published a Level 3 - Practice Enhanced Precautions regarding the Republic of Rwanda's MVD outbreak in October 2024. Furthermore, the CDC has issued various Travel Health Advisories focused on various Marburg outbreaks.
To protect the United States, the government launched health screenings for air travelers coming from the Republic of Rwanda in 2024. These Marburg virus-related screenings took place in Washington Dulles, Chicago O'Hare, and New York JFK airports.
The CDC advises caution when visiting an area with an active MVD outbreak.
Our Trust Standards: Medical Advisory Committee