Australia’s Japanese Encephalitis Outbreak Turns Fatal
The World Health Organization (WHO) reported Australia’s recent Japanese Encephalitis (JE) outbreak continues spreading unabated.
The first human JE case was reported in early March 2022 from Queensland.
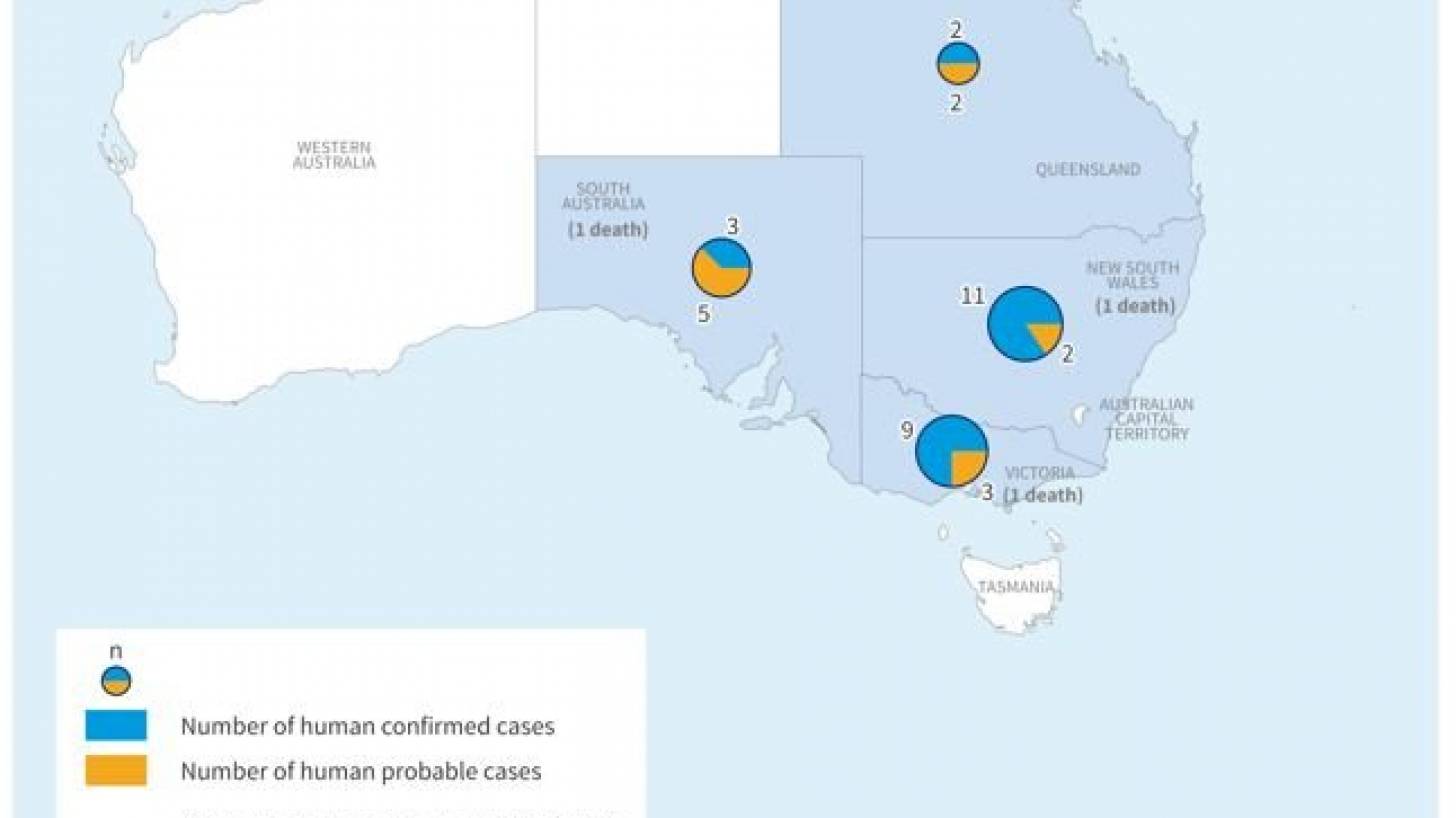
As of April 28, 2022, Australian health authorities reported a cumulative 37 human cases of Japanese encephalitis (25 laboratory-confirmed cases and 12 probable cases) and 3 related fatalities in four Australian states.
This outbreak represents the first locally-acquired JE cases detected on the Australian mainland since 1998.
The number of JE cases and deaths reported in 2022 is unusually high as compared to the previous ten years.
The WHO says the current events represent a significant change in the presence of the virus in Australia.
The Australian Government has declared the JE outbreak a Communicable Disease Incident of National Significance under the Emergency Response Plan for Communicable Disease Incidents of National Significance.
Although a rare disease in humans, JE is a serious viral infection caused by the JEV spread by infected Culex spp. Mosquitos. But, JEV cannot be transmitted from human to human, or by consuming meat from an infected animal.
However, in an immunologically naïve population, all age groups are at risk for JEV infection.
There are no treatments for JE and the case fatality rate among symptomatic cases can be as high as 30%. Permanent neurologic or psychiatric sequelae can occur in 30–50% of cases with encephalitis.
Local transmission of JEV to humans requires environmental conditions capable of sustaining an enzootic cycle. Thus, the risk of infection can vary substantially within any endemic country.
JE transmission does intensify during rainy seasons, during which vector populations increase.
Internationally, there has not yet been evidence of increased JEV transmission following major floods or tsunamis.
This means, that the risk at the regional and global level is assessed as low by the WHO.
The WHO recommends the implementation of activities to remove potential mosquito breeding sites, reduce vector populations and minimize individual exposures, including vector control strategies.
Homes near pig farms or animal shelters should be protected with mosquito-proof screens on windows and doors.
Furthermore, the WHO encourages vaccine strategies should be designed and implemented.
Due to the need for rapid production of protective antibodies, rapid deployment of at least one dose of live attenuated or live recombinant vaccines should be used.
And JE vaccination should be integrated into national immunization schedules in all areas where JE is recognized as a public health priority.
There are two JE vaccines for humans registered for use in Australia only advised for risk groups.
France-based Valneva SE's IXIARO is the only JEV preventive vaccine approved by the U.S. FDA. Currently, Ixiaro is licensed in Australia, where it is marketed as JESPECT®.
Ixiaro is an inactivated, adsorbed Vero cell culture-derived vaccine developed through a cooperative agreement with the Walter Reed Army Institute of Research. It is prepared by propagating JEV strain SA14-14-2 in Vero cells.
Unfortunately, immunocompromised individuals may have a diminished immune response to IXIARO.
Vaccination appointments for Ixiaro and other travel vaccines can be requested at this weblink.
Moreover, the WHO advises against applying any travel or trade restrictions based on the current information available on this event.
On March 22, 2022, the U.S. CDC issued a Level 2 Travel Advisory regarding Australia’s JEV outbreak.
Vax-Before-Travel publishes fact-checked research-based travel vaccine news.
Our Trust Standards: Medical Advisory Committee