Trivalent Poliovirus Virus-like Particle Vaccines May Solve Shedding Concern

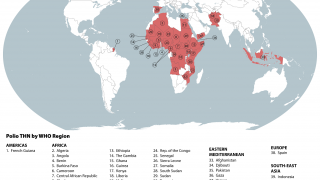
Following the launch of the Global Polio Eradication Initiative in 1988, the number of paralytic poliomyelitis cases was reduced by about 99% globally, with wild-type PV1 remaining endemic in only Afghanistan and Pakistan.
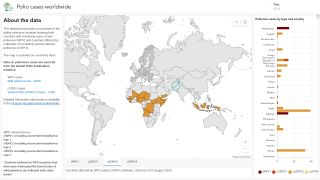
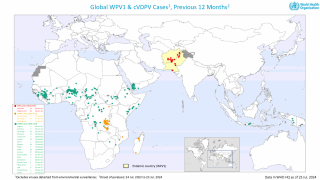
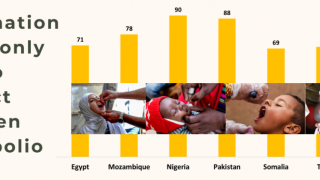
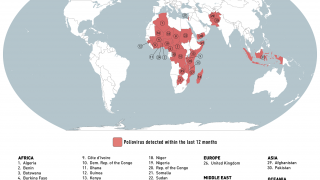
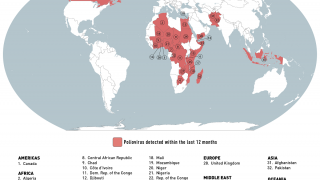
However, the World Health Organization recently reconfirmed that the spread of the poliovirus remained a Public Health Emergency of International Concern. For example, the U.S. CDC identified polio outbreaks and poliovirus detections in 39 countries in 2025.
To address these concerns, a recent study highlighted the benefits of an innovative polio vaccine candidate.
According to a study published by NPJ Vaccines on March 31, 2025, the success of the poliovirus (PV) vaccines has enabled the near-eradication of wild PV. Their continued post-eradication use poses concerns due to the 'potential for virus escape during vaccine manufacture.'
While the current generation of PV vaccines has achieved great success, the continued use of oral PV has facilitated the continued appearance of circulating vaccine-derived PV (cVDPV), which now outnumbers wild PV cases yearly.
These researchers wrote, 'Recombinant virus-like particles (VLPs) that lack the viral genome remove this risk.'
They demonstrate the production of PV VLPs for all three serotypes by controlled fermentation using Pichia pastoris.
The cryo-EM structure of a new PV2 mutant, SC5a, was determined compared to PV2-SC6 b VLPs described previously, and the immunogenicity of PV2-SC5a VLPs was investigated.
Finally, a trivalent immunogenicity trial using bioreactor-derived VLPs of all three serotypes in the presence of Alhydrogel adjuvant showed that these VLPs outperform the current IPV vaccine in the standard vaccine potency assay, offering the potential for dose-sparing.
Overall, 'these results provide further evidence that yeast-produced VLPs have the potential to be a next-generation polio vaccine in a post-eradication world.'
'The most important pre-requisite of any next-generation PV vaccine is that it elicits the same long-lasting immunity against disease as the current vaccine.'
Over the past few years, PT Biofarma has produced the type 2 novel oral polio (nOPV2) vaccine, derived from the live, infectious virus and 'triple-locked.' The nOPV2 vaccine is reported to be more genetically stable than previous oral polio vaccines, with a lower risk of reversion to neurovirulence.
It has been administered over 1.1 billion times, mainly in Africa.
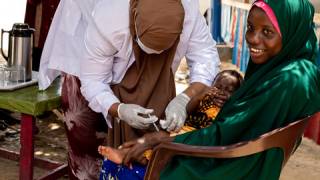
Since 2000, the IPV has been the standard polio vaccine offered in the United States, and booster doses are suggested when visiting poliovirus outbreak areas.
Our Trust Standards: Medical Advisory Committee