Tuberculosis Vaccine Candidate Launches Substantive Phase 3 Study
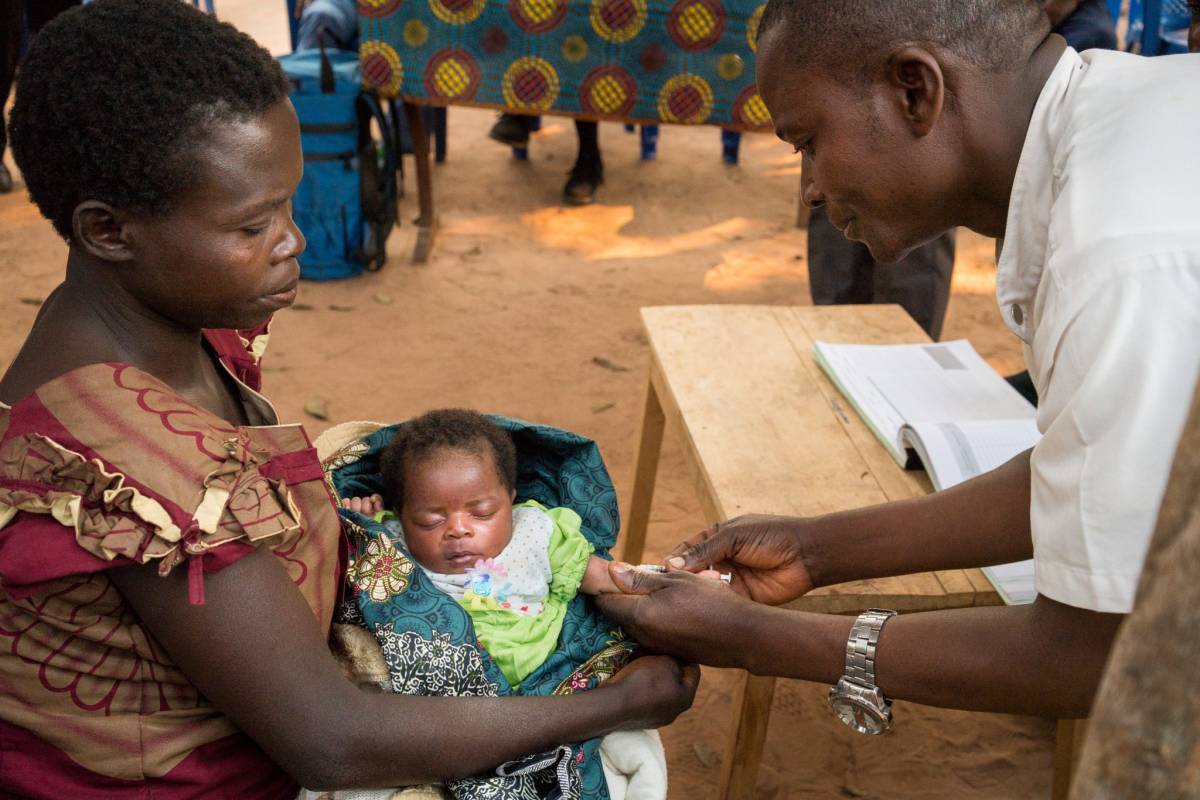
The Bill & Melinda Gates Medical Research Institute today announced that a Phase 3 clinical trial to assess the efficacy of the M72/AS01E tuberculosis (TB) vaccine candidate is now underway in South Africa.
At full capacity, the trial will include up to 20,000 participants, including people living with HIV, at up to 60 trial sites in seven countries.
If shown to be well-tolerated and effective, M72/AS01E could potentially become the first vaccine to help prevent pulmonary TB in adolescents and adults, the most common form of the disease.
Furthermore, M72/AS01E would be the first new TB vaccine in over a century.
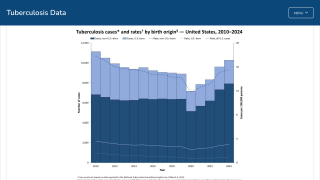
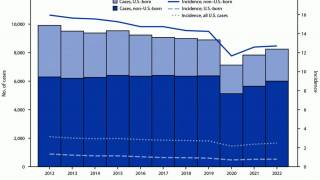
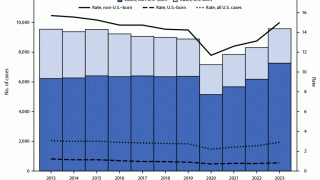
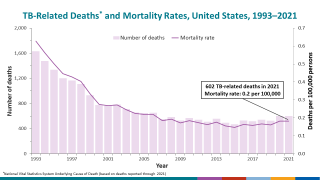
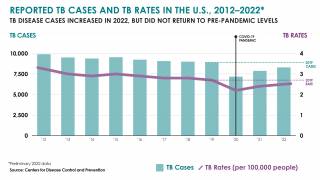
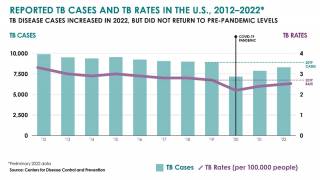
While TB is one of the world’s deadliest infectious diseases, the only available vaccine is Bacille Calmette-Guerin (BCG), which dates back to 1921.
BCG vaccines initially targeted against TB, tuberculosis meningitis, and non-specific protective effects against respiratory tract infections and certain cancers.
Various reports indicate that the BCG vaccine offers inadequate protection for adolescents and adults against the pulmonary form of the disease, which is primarily responsible for transmitting the TB bacterium.
Our Trust Standards: Medical Advisory Committee