Needle-Free Polio Immunization Study Launches
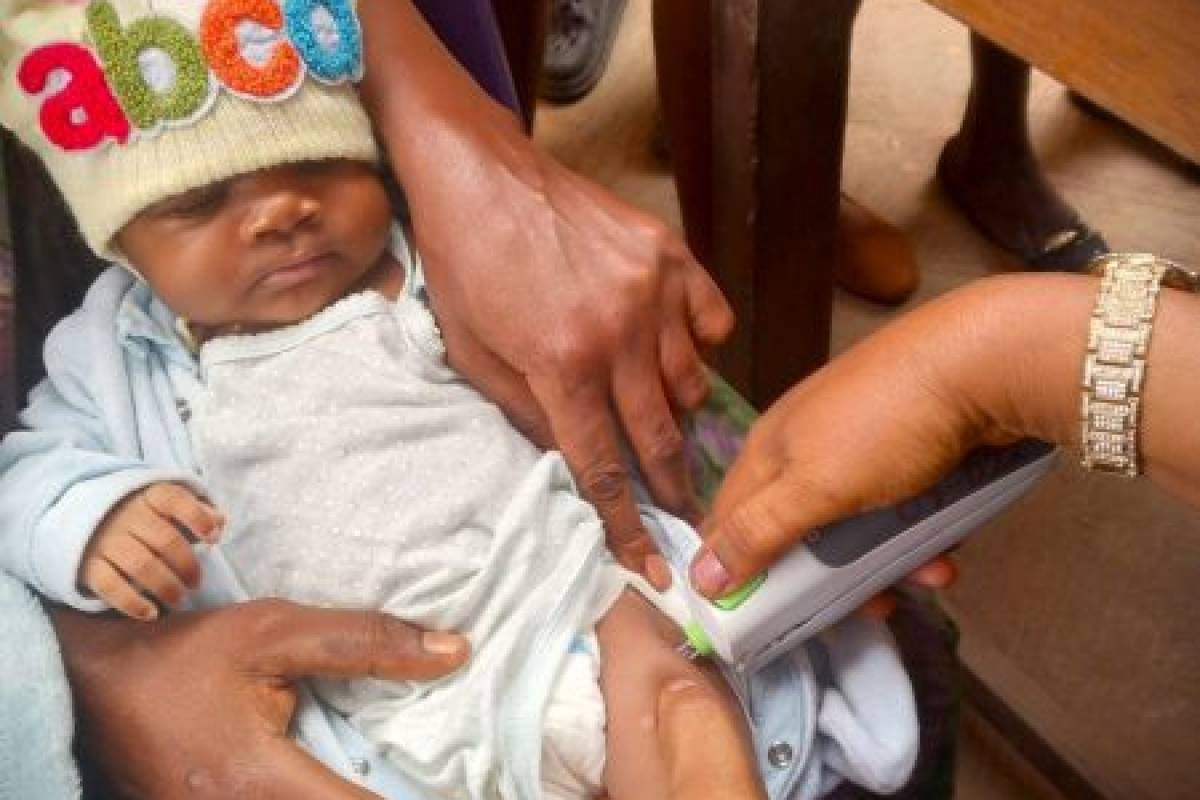
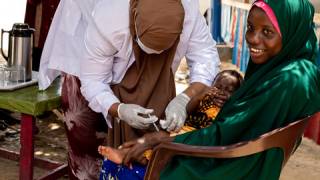
PharmaJet® recently announced the implementation of a study in Nigeria to evaluate the impact of intradermal vaccine administration of fractional inactivated poliovirus vaccine (fIPV) using their Tropis® ID Needle-free Injection System (NFIS).
Announced on October 24, 2023, the study, in collaboration with the National Primary Health Care Development Agency, Jhpiego, PATH, and the Sydani Group, is assessing coverage rates and potential cost reductions associated with using Tropis for fIPV delivery as compared to the current standard of intramuscular delivery of full dose IPV using needle and syringe.
Partners are also evaluating the acceptability and feasibility of using needle-free from the healthcare worker and caregiver perspective.
The study will run through January 2024, with children at 22 urban and rural health facilities receiving fIPV with Tropis. Evidence from the study is intended to inform policy regarding intradermal delivery of polio vaccine in routine immunization settings.
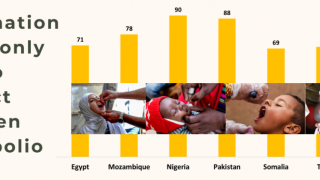
Paul LaBarre, Vice President of Global Business Development, PharmaJet, commented in a press release, “This (effort) builds on our experience in Pakistan where Tropis has been demonstrated to increase coverage rates. Including additional campaigns in Somalia, we have provided more than 10 million syringes for polio immunization programs."
"On this World Polio Day 2023, we restate our commitment to the Global Polio Eradication Initiative.”
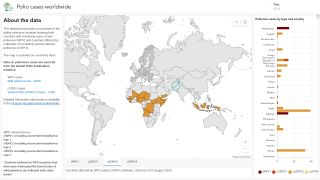
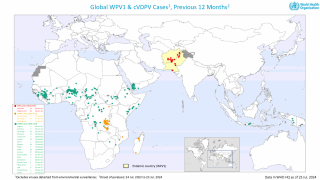
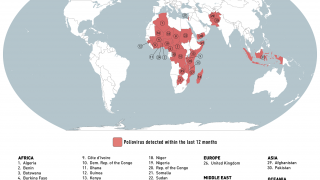
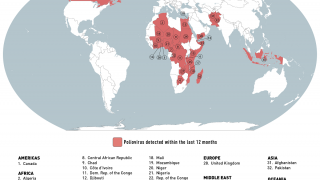
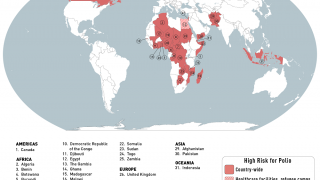
As of October 30, 2023, the U.S. CDC confirms over thirty countries have reported polio outbreaks in the past year.
Our Trust Standards: Medical Advisory Committee