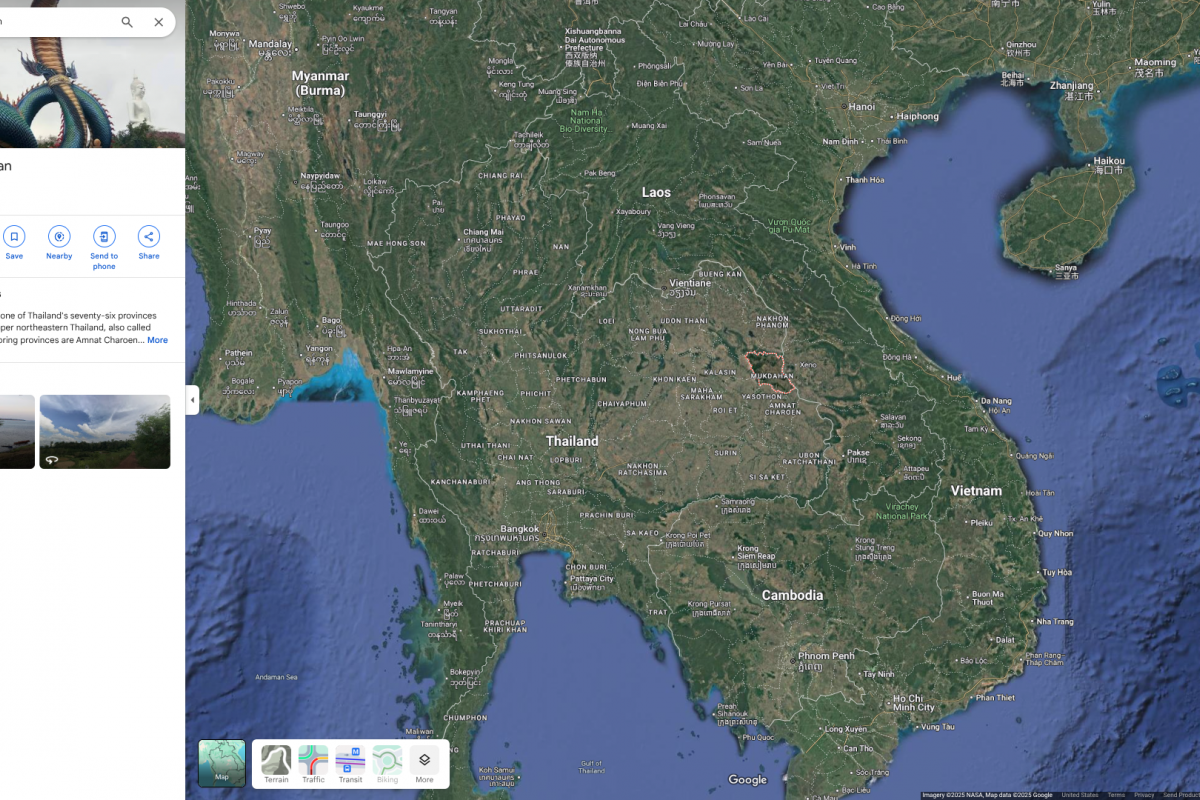
The Kingdom of Thailand has reported its first anthrax-related fatality since 1994, when a man in Mukdahan province died from the disease after being exposed to an infectious cow. All types of anthrax have the potential, if untreated, to spread throughout the body and cause severe illness and death.
According to the Health Ministry on May 2, 2025, they are tracking hundreds of people who may have been exposed to the bacteria. They found 247 people who were in contact with the disease, divided into 28 people who butchered the cattle and 219 people who consumed raw beef.
The Ministry has given medication to the high-risk contact group.
Located in Southeast Asia, Thailand is home to over 60 million people and is a vacationer's favorite destination in 2025.
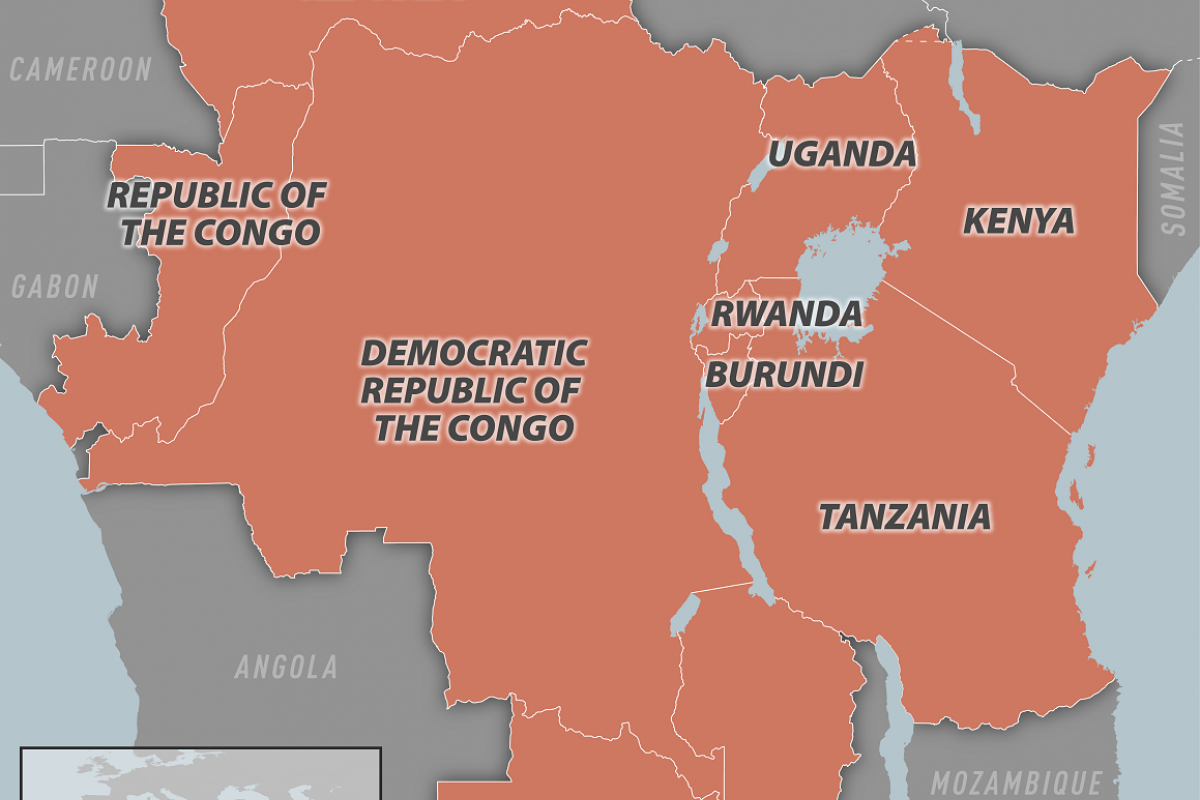
Last week, the Democratic Republic of Congo confirmed a human case in North Kivu Province.
Various types of anthrax are caused by a spore-forming bacterium called Bacillus anthracis. There are thought to be a few thousand human cases worldwide each year.
In the United States, Healthcare providers rarely see a patient with anthrax, says the U.S. CDC. Access to anthrax vaccines, such as CYFENDUS™, is controlled by the government.