Based on the updated data posted on Facebook from the South American country of Colombia, the government's yellow fever emergency is expected to continue into May 2025.
As of April 29, 2025, the Ministry of Health has reported 60 cases of yellow fever and 24 related fatalities this year.
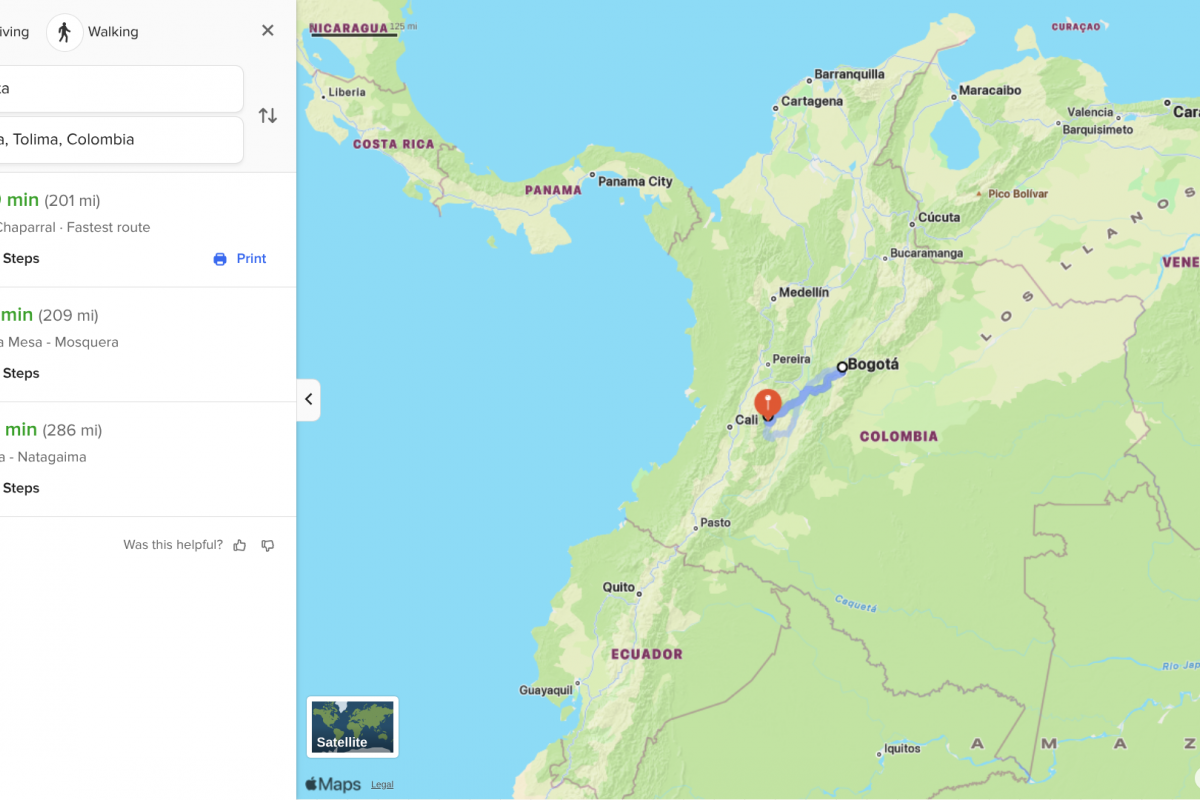
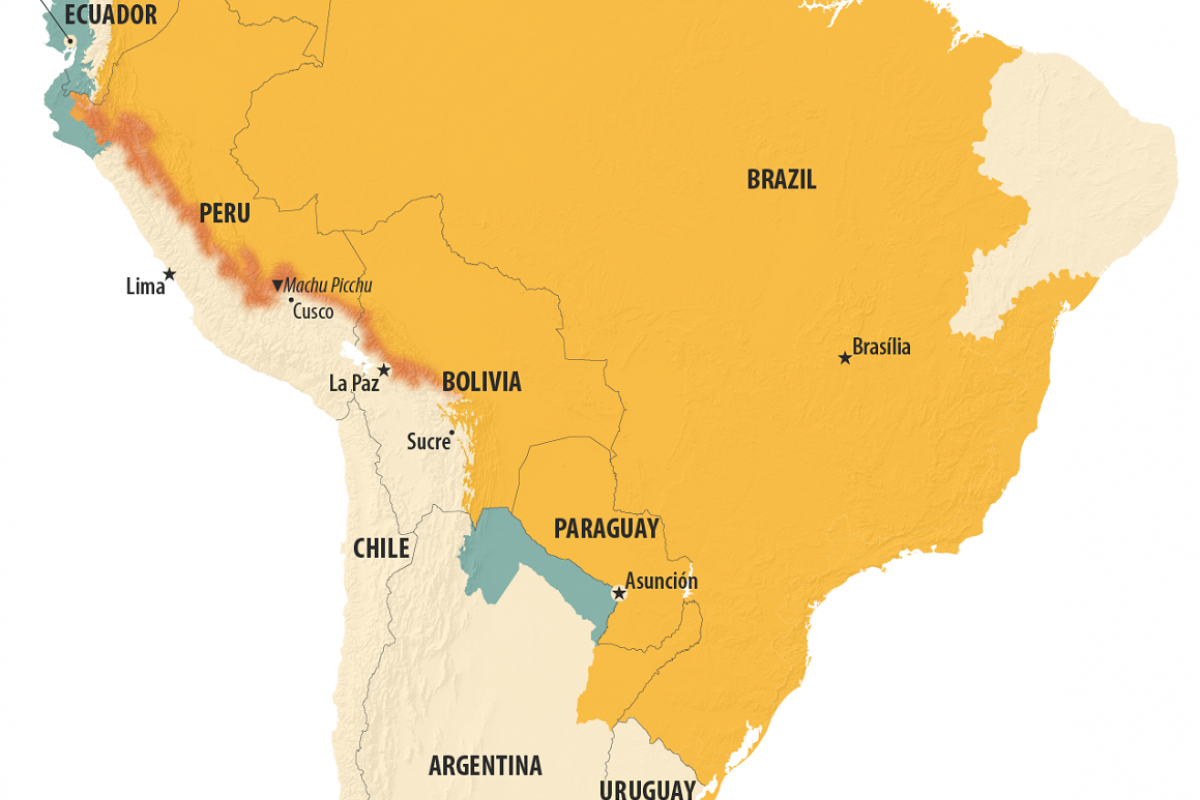
While ten departments have reported cases, Tolima and Putumayo are the most affected.
In Tolima, the outbreak has been particularly severe, with over 13 deaths in six months, representing a lethality rate of 41.9%.
Tolima is located about 200 miles southwest of Bogota, a city with over 7 million residents.
According to the U.S. Centers for Disease Control and Prevention (CDC) Level 2 Travel Health Advisory, yellow fever outbreaks have been reported in new areas in Brazil, Bolivia, and Peru in 2025.
Travelers to these newly affected areas are now recommended to get vaccinated, at least 10 days before travel.
The CDC writes, 'a booster dose may be given to certain travelers or travelers who received their last dose of yellow fever vaccine at least 10 years previously and who will be in a higher-risk setting.'
Since yellow fever is a vaccine-preventable disease, the YF-VAX® vaccine is commercially available at certified travel clinics and pharmacies in the United States.