Positive Breast Cancer Survival Data Presented

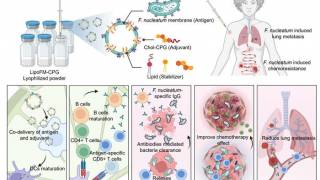
California based BriaCell Therapeutics Corp. announced the overall survival (OS) data of its lead product candidate, Bria-IMT™, in combination with Checkpoint Inhibitors in advanced breast cancer patients.
BriaCell stated on October 21, 2020, the median OS of 13.3 months has been observed in Phase I/IIa study for patients treated with Bria-IMT™ in combination with immune checkpoint inhibitors in patients with advanced breast cancer (third-line or later).
Furthermore, the median OS had reached 14-months in patients with Grade I/II tumors.
This data is derived primarily from patients previously disclosed on June 22, 2020. An OS of 7.2-9.8 months in similar patients with metastatic breast cancer in the third-line setting has recently been published (Kazmi S, et al. “Overall survival analysis in patients with metastatic breast cancer and liver or lung metastases treated with eribulin, gemcitabine, or capecitabine.” Breast Cancer Res Treat. 2020).
The checkpoint inhibitors included pembrolizumab (KEYTRUDA), and more recently, Incyte’s INCMGA00012.
The Combination Study is listed in ClinicalTrials.gov as NCT03328026.
BriaCell is an immuno-oncology focused biotechnology company developing targeted and effective approaches for the management of cancer.
Vax-Before-Cancer publishes research-based oncology news.
Our Trust Standards: Medical Advisory Committee