Plant-Based Dengue Virus Vaccine Identified

Researchers from the United Kingdom announced they used plants to produce virus-like particles (VLPs) of the dengue virus in a potential first step towards novel vaccines against the growing threat.
Led by Professor George Lomonossoff at the John Innes Centre in collaboration with Leaf Expression Systems published a pre-clinical study on October 25, 2020, which produced VLPs of dengue virus serotype 1, which is one of 4 serotypes of the virus.
These researchers used a variety of tobacco (Nicotiana benthamiana) to transiently express the VLPs using technology pioneered at the John Innes Centre by Professor Lomonossoff. After extraction and purification, the VLPs were shown to stimulate an immune response to the virus in experiments on mice.
“This is the first of what would be many steps along the path towards making dengue vaccines in plants,” said corresponding author Dr. Hadrien Peyret of the John Innes Centre, in a press release issued on December 2, 2020.
Using the metabolism of plants to produce these valuable molecules potentially offers an affordable and low-tech solution to vaccine development.
VLPs are authentic mimics of the virus containing the protein coat but without the infectious material. This as the basis for a vaccine is enough to train the immune system without causing an infection.
Virus-like particles for non-enveloped viruses can, in most cases, be readily produced in plants using transient expression technology. It was also known that VLPs of the enveloped influenza virus could be produced in plants using this technology.
However, influenza VLPs seemed to be an exception, for this type of virus with a protective lipid outer-envelope. Until this study, there were no published examples of VLPs purified from plants for other enveloped viruses.
“What we’ve made is not a candidate dengue vaccine: at best it’s a quarter of a candidate dengue vaccine. But for experts in this field, the exciting aspect is the production and purification of enveloped VLPs in plants without using the influenza virus HA transmembrane domain which would have been an alternative strategy,” added Dr. Peyret.
“For non-experts, it’s exciting that plants increasingly look like an interesting tool for vaccine production. It might not always be the right tool for the job, but it is handy to have just in case,” he added.
Despite the promise underlying the research, there is much work to do before researchers can achieve the aim of producing a dengue vaccine candidate from plants.
A potential VLP-based dengue vaccine would need to contain all four serotypes to be effective.
Yields of VLPs (how much is obtained from each plant) was also much lower than normally expected from non-enveloped viruses.
This means it would take a substantial number of plants to produce just a few doses unless the team improves how much the plants produce and/or how they extract the VLPs from the plants.
Dr. Peyret commented: “The extraction and purification process was painstaking – we hadn’t anticipated quite just how difficult it would be. Also, we were surprised that we failed to get VLPs for the other serotypes. We have some ideas as to why that might be, but it was still surprising that all the hard work was only enough for serotype 1.”
Further work will focus on improvements to the methods and extraction of the VLPs and trying to replicate this for the other serotypes of the dengue virus.
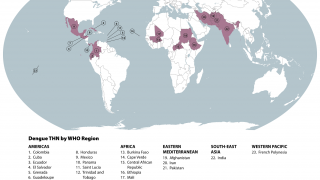
Dengue is a pathogenic mosquito-borne virus belonging to the Flaviviridae family that causes 390 million infections per year. The dengue virus complex consists primarily of four different dengue virus serotypes, which are roughly 65% genetically identical to each other and all members are transmitted by Aedes species mosquitoes, specifically, Aedes aegypti and Aedes albopictus, says the Mayo Clinic.
In Severe Dengue cases, fatalities can occur.
Dengue is common in the US territories of Puerto Rico, the US Virgin Islands, and American Samoa, says the U.S. CDC.
In south Florida and California, dengue cases are most often related to international travelers. However, beginning in 2019, the Key West area in south Florida reported an outbreak of locally-acquired dengue fever cases.
The U.S. FDA authorized the Dengvaxia vaccine for limited use in the USA on May 1, 2019. Dengvaxia should be used by people with laboratory-confirmed previous dengue infection and living in endemic areas.
PrecisionVaccinations publishes research-based vaccine development news.
Our Trust Standards: Medical Advisory Committee