Acute Flaccid Myelitis Cases Confirmed During 2019

There have been 7 Acute Flaccid Myelitis (AFM) cases confirmed during 2019 by the Centers for Disease Control and Prevention (CDC) on February 4, 2019.
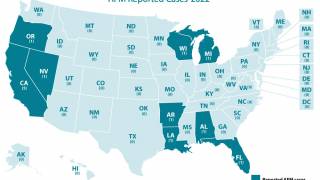
The CDC updated its year-end report for 2018 saying 210 AFM cases were confirmed in 40 states.
This year-end total may change since there are still 157 PUIs (patients under investigation) during 2018 still under investigation by the CDC and state and local health departments.
The leading states reporting confirmed AFM cases during 2018 were Texas (29), Colorado (16), Ohio (13), Washington (11), California (11), Minnesota (10), and New Jersey (10).
This new information compares with 2017 when the CDC confirmed just 35 ARM cases from 16 states.
in 2016, the European Center for Disease Control and Prevention was informed that Denmark, France, the Netherlands, Spain, Sweden, and the UK reported clusters and isolated cases of severe neurological syndromes in children and adults associated with enterovirus infection among which EV-D68 was detected.
And, in October 2017, the Argentina International Health Regulations National Focal Point reported a cluster of AFM associated with EV-D68 infection.
AFM is a complex condition that is diagnosed by examining a patient’s nervous system in combination with reviewing pictures of the spinal cord, says the CDC.
AFM symptoms are similar to other neurological diseases, such as Guillain-Barre syndrome (GBS), acute disseminated encephalomyelitis (ADEM), and transverse myelitis.
AFM is difficult to determine why only some people go from having a mild respiratory illness (90%) to developing AFM, says the CDC.
The CDC says it has tested different specimens from AFM patients for a wide range of pathogens and viruses.
There is no specific treatment for AFM, but a doctor who specializes in treating brain and spinal cord illnesses (neurologist) may recommend certain interventions on a case-by-case basis.
For example, surgeons at Children's Hospital Los Angeles have reported some success with nerve transfer surgery in children after AFM-induced paralysis.
Further, the CDC published an updated “Interim Considerations for Clinical Management of Patients with AFM” in November 2018, which includes the following statements:
- There is no indication and that any specific targeted therapy or intervention should be either preferred or avoided in the treatment of AFM.
- There are currently no targeted therapies/interventions with enough evidence to endorse or discourage their use for the treatment or management of AFM.
- Clinicians should expedite neurology and infectious disease consultations to discuss treatment and management considerations.
- There is no indication that corticosteroids should be either preferred or avoided in the treatment of AFM. There is no clear human evidence for the efficacy of steroids in the treatment of AFM, and there is some evidence in a mouse model with EV-D68 that steroids may be harmful. The possible benefits of the use of corticosteroids to manage spinal cord edema or white matter involvement in AFM should be balanced with the possible harm due to immunosuppression in the setting of a possible viral infection.
- There is no indication that IVIG should be either preferred or avoided in the treatment of AFM. There is no clear human evidence for the efficacy of IVIG in the treatment of AFM; evidence for efficacy is based on early treatment in animal models and it has not been given in a systematic manner to AFM patients to allow for measurements of efficacy. There is no evidence that treatment with IVIG is likely to be harmful.
- There is no indication that plasma exchange should be either preferred or avoided in the treatment of AFM. There is no clear human evidence for the efficacy of plasma exchange in the treatment of AFM, and it has not been given in a systematic manner to AFM patients to allow for measurements of efficacy. Although there are inherent procedure-associated risks, there is no evidence that using plasma exchange for patients with AFM is likely to be harmful.
- There is no indication that fluoxetine should be used for the treatment of AFM. There is no clear human evidence for the efficacy of fluoxetine in the treatment of AFM based on a single retrospective evaluation conducted in patients with AFM, and data from a mouse model also did not support efficacy.
- There is no indication that antivirals should be used for the treatment of AFM, unless there is suspicion of herpesvirus infection (e.g., concomitant supra-tentorial disease or other clinical or radiologic features of herpesvirus infection). Appropriate antiviral medications (i.e., acyclovir, ganciclovir) should be empirically administered until herpesvirus infection has been excluded.
- There is no indication that interferon should be used for the treatment of AFM, and there is concern about the potential for harm from the use of interferon given the immunomodulatory effects in the setting of possible ongoing viral replication.
- There is no indication that biologic modifiers and the use of other immunosuppressive agents should be used for the treatment of AFM, and there is a possibility of harm in their use.
Our Trust Standards: Medical Advisory Committee
- AFM Investigation
- Epidemiological Alert - Acute Flaccid Myelitis associated with enterovirus D68 in the context of Acute Flaccid Paralysis
- Acute flaccid myelitis
- 201 AFM Cases Confirmed During 2018
- An increase in reports of acute flaccid paralysis (AFP) in the United Kingdom, 1 January 2018–21 January 2019: early findings