Children May Need More Than One Yellow Fever Vaccination
In 2013, the World Health Organization (WHO) withdrew its recommendation for the yellow fever (YF) vaccine booster doses. However, new research supports the Brazilian National Immunization Program's recommendation for a booster dose of the vaccine at four years of age.
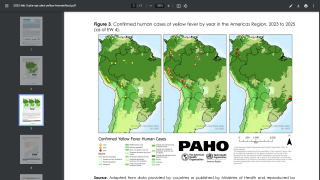
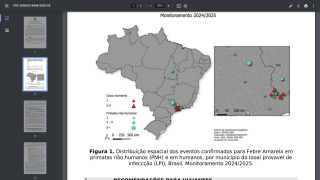
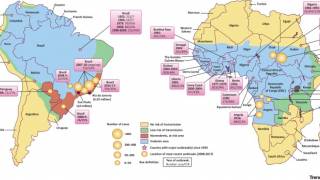
This vaccination policy change is vital for the Federative Republic of Brazil as it combats the spread of this severe mosquito-transmitted virus.
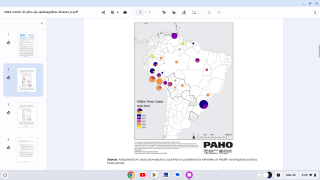
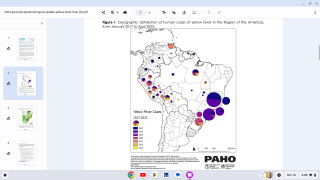
To assess the duration of vaccine-induced immunity, Plos Neglected Tropical Diseases published research on April 9, 2025, of a study cohort involving adults and children in an area in northeastern Brazil without wild-type YF virus circulation, but with a high incidence of other mosquito-transmitted orthoflavivirus, such as Zika.
This phase IV, uncontrolled cohort study was carried out in three municipalities in northeastern Brazil.
The 17DD strain of the (YF-VAX, Stamaril) vaccine was administered to children aged 9 months to 4 years and adults aged 18 to 50 years. Blood samples were collected for antibody titration before vaccination, 30–45 days after vaccination, and one year post-vaccination.
This study showed that YF seroconversion rates 30–45 days after vaccination increased with age, reaching 99% in adults. However, 10% of infants did not have detectable antibodies.
These findings are the first to examine a cohort of vaccinees with serological follow-up in an area without YF viral circulation.
In summary, these researchers wrote, 'Current recommendations assuming lifelong protection from a single dose of the YF vaccine do not appear to provide sufficient protection in high-risk areas, particularly where infants are the primary target for vaccination.'
These findings align with a previous cross-sectional study, which found that 76.4% of children were seropositive between 9 and 23 months after vaccination. In addition to showing lower seroconversion rates, children aged 9–12 months had significantly lower antibody titers over time, with seropositivity dropping below 60% four years after vaccination.
According to the U.S. Centers for Disease Control and Prevention (CDC), yellow fever vaccination is recommended for people aged nine months or older who are traveling to or living in areas at risk for the YF virus, such as Brazil and Colombia.
The 17D yellow fever vaccine was initially approved for human use in 1938, and about 850 million doses have been distributed..
In the United States, the YF-VAX® vaccine is commercially available at travel clinics and pharmacies in April 2025.
Our Trust Standards: Medical Advisory Committee