West Nile Virus Heats-Up in Arizona

According to the Arizona Department of Health (ADH), the number of human West Nile Virus cases has already surpassed 2018’s total of 24 cases.
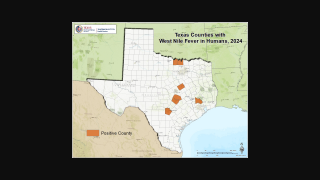
The ADH reported as of July 5, 2019, there were 27 West Nile Virus (WNV) cases in Maricopa County, which includes the city of Phoenix, Arizona.
This new data indicates Maricopa County, with a population of just 4.3 million, has reported 69 percent of the WNV cases in the USA during 2019.
As of July 9, 2019, a total of 39 human cases of WNV disease have been reported to the US Centers for Disease Control and Prevention (CDC).
This is unfortunate news since there is no cure nor preventive vaccine available for the WNV disease.
Of the WNV cases reported to the CDC during 2019, 62 percent were classified as neuroinvasive diseases, such as meningitis or encephalitis.
Previously, researchers identified 2 dominant strains of WNV — NA/WN02, and SW/WN03 — which have circulated in Maricopa County for approximately 4 and 7 years, respectively.
"You can expect we are going to see a lot more WNV cases than we've seen in the last five years," said Rebecca Sunenshine, Maricopa County Department of Public Health, in a press release.
‘The most effective way to prevent WNV infections is to prevent mosquito bites.’
“If you want to know if you live in a high-risk mosquito area, or if your neighborhood has been treated for mosquitoes recently, Maricopa County publishes an online map with those details.’
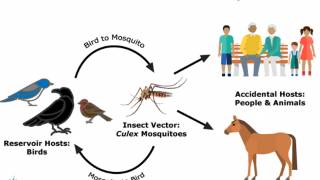
The first Arizona WNV case appeared in 2003, four years after it emerged in the USA, most likely from bird migrations.
A February 2019 study suggested Arizona’s moderate temperatures enables the WNV to survive over-winter in the Grand Canyon State in populations of mosquitoes and birds.
As a result, these researchers believe Arizona is a likely permanent source of WNV infection that is annually exported by migratory birds to other states.
Additionally, the CDC publishes a nationwide map of reported WNV cases.
During 2018, the CDC confirmed 2,544 WNV cases and 137 related fatalities in the USA.
West Nile virus
WNV, the leading cause of mosquito-borne disease in the continental United States, was identified in America in New York in 1999, says the CDC.
West Nile virus is neurotropic mosquito-borne flaviviruses within the family Flaviviridae. The genus Flavivirus is comprised of more than 70 recognized viruses, including some of the most significant arboviral pathogens of humans.
WNV is an enveloped positive-stranded ribonucleic acid virus, belonging to the Japanese encephalitis serocomplex.
Symptoms
According to the CDC, 80 percent of people infected with West Nile virus do not develop any symptoms.
But, about 20 percent of people who are infected develop a fever, with other symptoms such as headache, body aches, joint pains, vomiting, diarrhea, or rash.
Most people recover completely, but fatigue and weakness can last for weeks or months.
Severe illness can occur in people of any age; however, people over 60 years of age are at greater risk. People with certain medical conditions, such as cancer, diabetes, hypertension, kidney disease, and people who have received organ transplants, are also at greater risk.
The recovery from severe illness might take several weeks or months.
Diagnosis
Reporting WNV cases in a timely manner is important. According to a study published in the JAMA Network Open in April 2019, discovered that reporting human WNV cases were delayed by 2 to 14 weeks.
These reporting delays prevented health departments from notifying the local community to take preventive actions in a timely manner.
See your healthcare provider if you develop the symptoms described above. Your healthcare provider can order tests to look for West Nile virus infection.
To learn more about testing, visit the Healthcare Providers page.
Treatment
There is not a therapeutic vaccine or specific antiviral treatments for West Nile virus infection available.
Over-the-counter pain relievers can be used to reduce fever and relieve some symptoms
In severe cases, patients often need to be hospitalized to receive supportive treatment, such as intravenous fluids, pain medication, and nursing care.
If you think you or a family member might have West Nile virus disease, talk with your health care provider.
To learn more about treatment, visit the Healthcare Providers page.
Vaccine
Over the last two decades, several vaccine candidates for the protection of humans from WNV have been developed. Some technologies were transferred into clinical testing, but these approaches have not yet led to a licensed product.
To date, clinical trials have been performed with a DNA vaccine, chimeric flaviviruses using the yellow fever vaccine strain or an attenuated Dengue virus vaccine as backbones and a recombinant, insect-cell-derived E protein ectodomain.
In addition, two inactivated whole virus vaccine candidates have been evaluated clinically. Firstly, two doses of a hydrogen peroxide inactivated WNV vaccine led to detectable neutralizing antibodies in approx. 50% of the study participants. The authors indicate that this result might be improved by adding a third dose or by using an alternative inactivation protocol which combines hydrogen peroxide and formaldehyde. Secondly, three doses of formaldehyde inactivated WNV particles induced high titers of neutralizing antibodies.
There is one WNV vaccine candidate conducting a small phase 1 clinical trial. This vaccine, HydroVax-001, is a hydrogen peroxide inactivated, whole virion vaccine adjuvanted with aluminum hydroxide. HydroVax-001 experimental vaccine was discovered and developed by scientists at Oregon Health & Science University.
West Nile virus news
Our Trust Standards: Medical Advisory Committee