Dengue Vaccination Enters a New Age

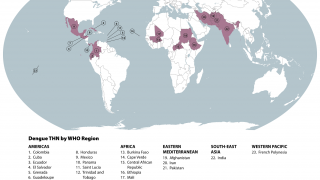
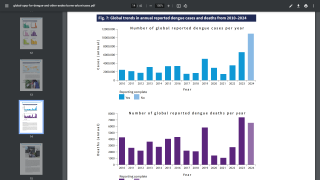
A Viewpoint published by the JAMA Network on December 15, 2021, highlights how dengue disease has become an increasing risk in various countries.
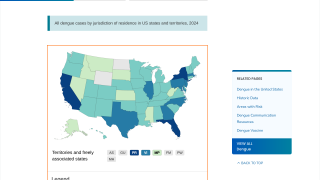
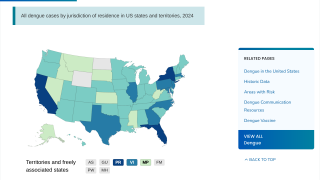
Furthermore, as the Aedes species mosquito vector reaches into new areas, the risk for large outbreaks in U.S. territories and gateway states increases daily as international travelers return from endemic regions.
These researchers say, ‘Clinicians should become familiar with practical dengue prevention tools such as vaccines.’
This Viewpoint summarizes the new recommendations of the U.S. CDC’s Advisory Committee on Immunization Practices (ACIP) for using Sanofi Pasteur’s Dengvaxia dengue vaccine, the first vaccine licensed by the U.S. Food and Drug Administration (FDA) to prevent dengue.
Dengue can affect people of all ages, although children and adolescents 10 through 19 are at the highest risk for severe disease and hospitalization in endemic areas. This occurs because primary and secondary dengue infections are associated with a higher risk for poor outcomes than subsequent dengue infections.
Most people have two or more dengue infections in endemic areas before adulthood.
To meet the growing challenge of dengue prevention and control, manufacturers and public-private consortia have worked for years to develop a pediatric dengue vaccine.
A generally accepted criterion for dengue vaccines is efficacy against all four dengue viruses, which stems from the increased risk for severe dengue associated with a second dengue infection occurring months to years after the initial infection.
This increased risk is related to antibody-dependent enhancement, in which low antibody levels are ineffective at neutralizing the virus but instead facilitate entrance into cells and increase the viral load, leading to plasma leakage and clinically significant extravascular fluid accumulation.
This risk became clear when Dengvaxia was introduced in the Philippines in 2016 among schoolchildren aged 9 to 10 years.
Following vaccination of about 800,000 children in this age cohort, the Philippines suspended the dengue vaccine program in 2017 when clinical studies found that children without previous dengue virus infection had increased risk for hospitalization (cumulative incidence of 15.7 vs. 10.9 per 1000 for seronegative vaccinated children and controls, respectively).
And severe dengue (cumulative incidence of 4.0 vs. 1.7 per 1000, respectively) risks increased after vaccination.
Although the vaccination period was approximately one year, the Philippines experienced an extensive public outcry against the dengue vaccine.
In response to the growing concerns over potential risks, the ACIP took about two years to weigh the benefits and risks of the dengue vaccine.
Finally, in voting on the Dengvaxia vaccine in June 2021, the ACIP recommended the vaccine for routine administration to children aged 9 through 16 living in dengue-endemic areas in the US and with laboratory confirmation of previous studies on dengue infection.
Endemic areas include more than 3.6 million people in Puerto Rico, the US Virgin Islands, American Samoa, the Federated States of Micronesia, the Republic of Marshall Islands, and the Republic of Palau, of which approximately 10% are in the age range recommended for vaccination.
Moreover, an important consideration behind the recommendation was the availability of an accurate test for prevaccination screening.
To ensure safety, the Centers for Disease Control and Prevention recommended that prevaccination screening tests have at least 98% specificity and 75% sensitivity.
Most previous dengue serologic tests were limited by either high cross-reactivity with other flaviviruses, such as the Zika virus, or were designed to identify high antibody titers in the days to weeks after acute dengue infection and lacked the sensitivity to detect low antibody titers months to years after infection.
Throughout 2021, evaluations conducted at CDC laboratories identified a laboratory-based 2-test algorithm with reflex testing that met the requirements for sensitivity and specificity, enabling the potential for accurate prevaccination screening to distinguish individuals who could benefit from vaccination from those without a previous dengue infection.
The development of precise point-of-care tests remains a priority.
Retail testing could facilitate accurate, rapid testing for previous dengue infections and allow same-day vaccination of eligible children.
Several fundamental challenges remain for the implementation of a dengue vaccine.
- First, communication to clinicians the necessary to explain that the vaccine can only be administered to children 9 to 16 years old who have laboratory evidence of previous dengue infection and live in endemic areas.
- Clinicians and parents should also be aware that the vaccine is not 100% effective against dengue and that breakthrough dengue cases are likely to occur among appropriately vaccinated patients.
- Additionally, endemic areas with low or infrequent dengue transmission may need to conduct dengue seroprevalence assessments among children aged 9 to 16 years before starting vaccination because it is not recommended in populations with low (<20%) seroprevalence due to the elevated risk for vaccinating children with false-positive results.
- And most importantly, clinicians must focus on the unintentional vaccination of children without previous dengue infection.
Logistical considerations also pose a severe challenge for the implementation of dengue vaccination.
- The prevaccination screening will likely require multiple visits to evaluate eligibility and initiate the vaccine series.
- Dengvaxia vaccination requires three doses at 6-month intervals, requiring reminders and an extended period before children are fully vaccinated.
The CDC’s recent recommendation for Dengvaxia starts a new era for dengue prevention.
For example, second-generation vaccine candidates are approaching regulatory approval. Takeda’s TAK-003 vaccine is currently in phase 3 clinical trials, with enhanced efficacy results registered.
This Viewpoint closes by saying, ‘Physicians practicing in dengue-endemic areas can take the first steps in promoting health equity by implementing screen-and-vaccinate programs among children 9 to 16 years old. Furthermore, physicians in all areas should be aware of new tools and possibilities in the rapidly developing field of dengue prevention.’
Corresponding Author: Laura E. Adams, DVM, MPH, Centers for Disease Control and Prevention, 1324 Calle Canada, San Juan, PR 00920 ([email protected]).
Vax-Before-Travel publishes fact-checked research-based dengue vaccine news.
Our Trust Standards: Medical Advisory Committee