$30 Million Awarded for Another Bird Flu Vaccine Candidate
A global leader in influenza prevention announced that the Biomedical Advanced Research and Development Authority (BARDA), part of the U.S. Administration for Strategic Preparedness and Response, had selected CSL Seqirus to manufacture and clinically assess a cell-based, adjuvanted pre-pandemic influenza vaccine candidate to support the U.S. government's pandemic preparedness activities.
Seqirus confirmed on October 6, 2022, that it intends to deliver an H5N8 A/Astrakhan virus vaccine candidate for assessment in a Phase 2 clinical study that is anticipated to begin in the second quarter of 2023.
This is the third influenza pandemic preparedness award granted to CSL Seqirus under a multi-year agreement with BARDA.
In the last twelve months, following an April 2022 award to produce an H5N8 A/Astrakhan cell-based synthetic influenza Working Seed lot, as well as a separate October 2021 award for developing one influenza A(H2Nx) vaccine virus candidate utilizing its cell-based and adjuvant technologies, and clinically assessing both the cell-based as well as its next-generation self-amplifying mRNA platform.
Previously, Seqirus's Audenz™ U.S. FDA Approval was in January 2020, receiving supplemental FDA approval in 2021.
Audenz is a monovalent, adjuvanted, cell-based inactivated influenza (H5N1) vaccine designed to protect individuals six months of age and older in the event of avian influenza (bird flu) pandemics.
Today's announcement further underscores the importance of public-private partnerships in bolstering pandemic preparedness.
Pre-pandemic influenza vaccines are developed for influenza strains with the pandemic potential to provide the first line of defense if a pandemic were to be declared.
Over the past year, outbreaks of highly pathogenic avian influenza (HPAI) A(H5) viruses among birds, commercial poultry, and animals have been reported throughout North America, as well as in Africa, Asia, and Europe.
The United States Department of Agriculture (USDA) says establishing HPAI in the U.S. is of great concern.
A widespread outbreak could have severe economic consequences for the poultry industry, and although transmission to humans has been rare, this highly mutable disease could pose a risk to humans.
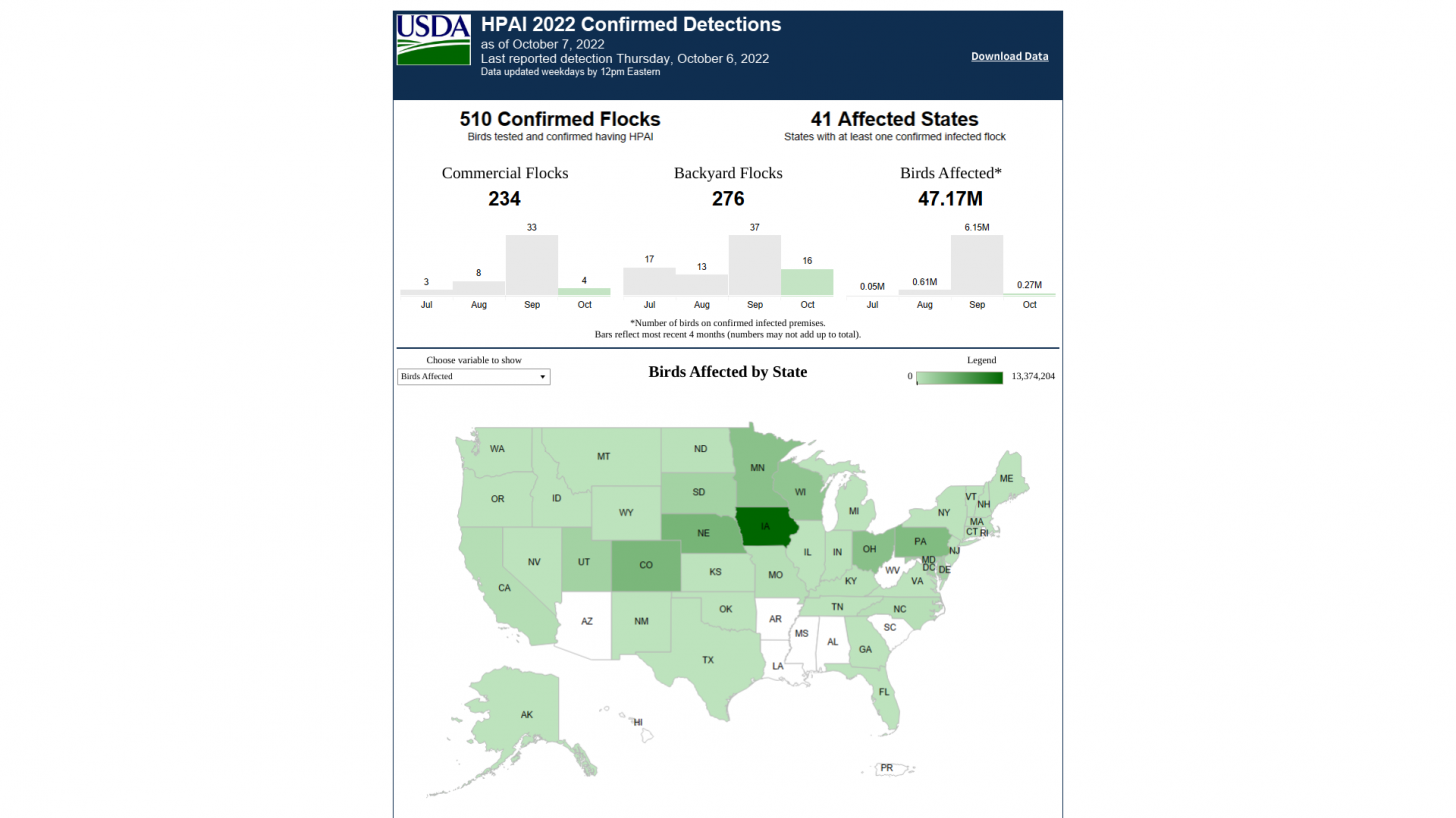
For example, the USDA reported the Eurasian H5N1 strain appeared in North America in January 2022 and has affected poultry/birds in 41 states and led to the loss of about 47 million birds as of October 7, 2022.
As of October 2022, the U.S. CDC says, the public health risk associated with these HAPI detections in birds remains low.
"When faced with a public health threat like highly pathogenic avian influenza, governments make plans to protect the population, which takes both time and a substantial collaborative effort with industry," commented Marc Lacey, Executive Director, Pandemic Response Solutions, CSL Seqirus, in a related press release.
"For more than a decade, CSL Seqirus has demonstrated its ability to come to the aid of government partners to prepare for and rapidly respond to emerging pathogens of concern, and we are able and ready to do so again for avian influenza."
Pandemic influenza is a contagious airborne respiratory disease but unlike the seasonal flu.
The risk of influenza-associated morbidity and mortality is greater with pandemic influenza because there is likely little or no pre-existing immunity to the virus in the human population.
Four influenza pandemics have occurred over the past century, with the 1918 pandemic being the most severe in recent history, estimated to have killed up to 50 million people worldwide.
This new CSL Seqirus vaccine project is supported in whole or in part with federal funds from the Department of Health and Human Services; Administration for Strategic Preparedness and Response; BARDA, under contract number 75A50122D00004.
Recently, the U.S. government awarded California-based Vir Bio a multi-year contract, with the potential for up to $1 billion to advance the development of a full portfolio of solutions to address influenza and potentially other infectious disease threats.
And the European Commission signed a framework contract on July 28, 2022, for the joint procurement of GSK's Adjupanrix, a pandemic influenza vaccine, under which the Member States can purchase up to 85 million vaccine doses if necessary, in the event of an influenza pandemic.
These vaccines may be needed in the future as the U.S. FDA says annual flu shots do not protect people from avian influenza (bird flu) pandemics.
Additional avian influenza outbreak news is posted at PrecisionVaccinations.com/Avian.
PrecisionVaccinations publishes fact-checked, research-based vaccine news manually curated for mobile readership.
Our Trust Standards: Medical Advisory Committee
























