Yellow Fever Vaccine Candidate Showed Optimal Viremia, Safety, and Immunogenicity

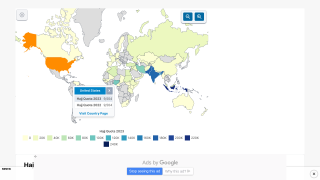
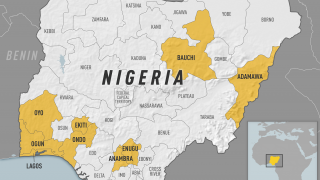
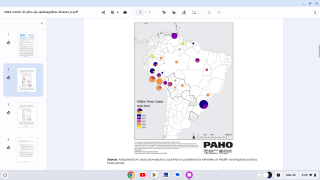
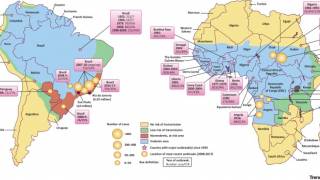
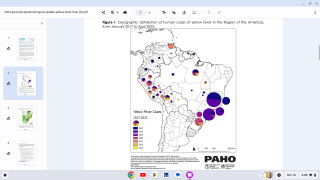
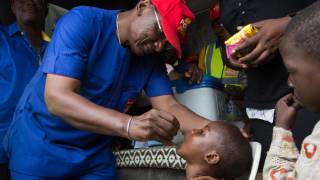
In 2024, yellow fever outbreaks remain a health threat in tropical regions of Africa and South America. The good news is that vaccines have been proven safe and effective for protecting international travelers visiting these areas.
However, new yellow fever vaccines with improved production scalability and enhanced efficacy are needed to reduce outbreaks.
The Lancet Infectious Diseases recently published results from a first-in-human phase 1 study on the safety and immunogenicity of a new Vero cell line-derived yellow fever vaccine, vYF-247.
Produced by Sanofi, the vYF-247 vaccine showed similar safety and immunogenicity to the U.S. FDA-approved YF-VAX vaccine.
These researchers concluded that the vYF-247 vaccine with a 5 Log CCID50 dose showed optimal viremia, safety, and immunogenicity and was chosen for further development.
Until a new vaccine is approved, the YF-VAX® vaccine remains available at travel clinics and pharmacies in the United States. For those travelers who were already vaccinated, the U.S. CDC says yellow fever vaccine booster doses are unnecessary.
Our Trust Standards: Medical Advisory Committee