Non-Invasive Bladder Cancer Test Granted U.S FDA Breakthrough Device Destination
California-based Nonagen Bioscience Corp today announced Oncuria™, their non-invasive bladder cancer test capable of predicting response to therapy, has been granted U.S. Food and Drug Administration (FDA) Breakthrough Device Designation.
This FDA designation is granted to technologies that have the potential to provide more effective treatment, diagnosis, or prognosis of life-threatening diseases, such as cancer.
The designation enables close collaboration with an expedited review by the FDA and formally acknowledges Oncuria's utility and potential clinical benefit.
"Oncuria is designed to provide a prediction of response to therapy, allowing for timely interventions that could result in more favorable outcomes for our patients," stated Charles J. Rosser, CEO of Nonagen, in a related press statement.
"We are proud that the FDA has decided to grant Breakthrough Device Designation to our lead diagnostic, Oncuria™, acknowledging growing recognition of the benefit our test can offer to clinicians and patients."
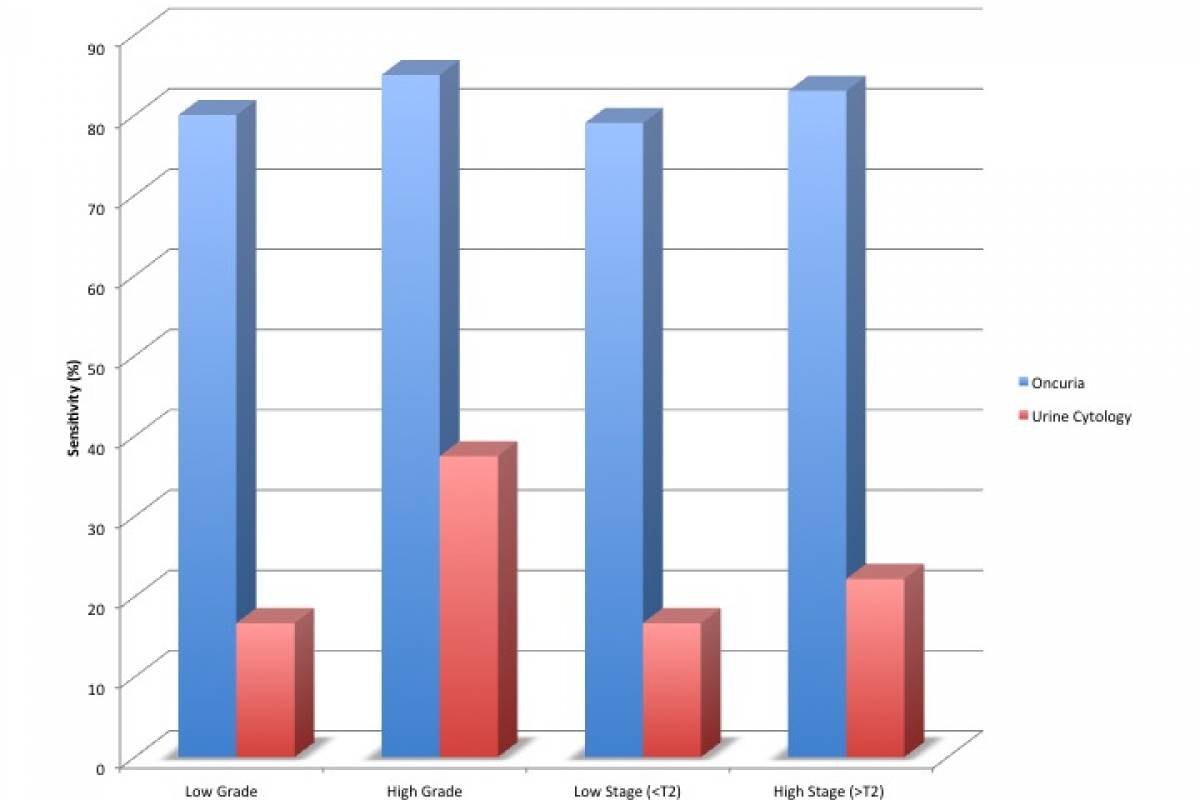
Oncuria is reported more sensitive for bladder cancer detection than urine cytology for both disease stage and by grade, says the Company.
Using a well-established Luminex Corporation platform, we are close to adding a robust diagnostic test in our fight against bladder cancer. Please take a look at our white paper to understand our journey and see how we stack up to competitors, says the Company's website.
Our Trust Standards: Medical Advisory Committee























