Search API
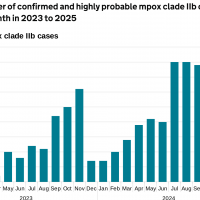
The World Health Organization (WHO) today published the 51st situation report for the multi-country outbreak of mpox, including an update on the epidemiological situation for mpox in Africa, with data as of late April 2025.
This year, the WHO has confirmed 137,892 mpox cases, 317 related fatalities, from 132 reporting countries.
The WHO stated on April 29, 2025, 'Wherever mpox outbreaks are not quickly contained and human-to-human transmission is not stopped, they continue to represent a potential risk of sustained transmission in the community.'
Highlights from this report include, but are not limited to, the following:
Cases of mpox due to clade Ib monkeypox virus (MPXV) continue to be reported primarily in Africa, where eleven countries have reported community transmission of this strain in the past six weeks, as person-to-person transmission has occurred through various means during this outbreak.'
Currently, Uganda is reporting the highest number of confirmed mpox cases globally, with 200 to 300 new cases reported per week. To date, the country has detected only clade Ib MPXV.
The Democratic Republic of the Congo (DRC) continues to report the highest number of cumulative confirmed mpox cases in Africa in 2025, despite a decrease in the number of confirmed cases reported in recent weeks, likely due to a reduction in testing and confirmation capacity. Clades Ia and Ib MPXV continue to circulate in the DRC.
This report provides an overview of mpox vaccination in countries in the African Region, where to date more than 662,000 doses of Bavarian Nordic's JYNNEOS® (MVA-BN®) vaccines have been administered in seven countries.
From the total number of doses, 88% have been administered in the DRC, where the vaccination strategy is being revised in light of the limited vaccine supply.
In the United States, there is an ample supply of the JYNNEOS vaccine, which is recommended by the U.S. CDC for specific individuals when visiting mpox outbreaks in 2025. This mpox vaccine is commercially available in the U.S. at clinics and pharmacies.

The U.S. Centers for Disease Control and Prevention (CDC) today confirmed an ongoing outbreak of clade I mpox in Central and Eastern Africa. Previous data indicates that about 15 million people visit central Africa each year.
As of April 1, 2025, the CDC updated its Level 2 Travel Health Advisory saying, 'There is ongoing person-to-person transmission of mpox in Burundi, Central African Republic, Democratic Republic of the Congo (DRC), Kenya, the Republic of the Congo, Rwanda, Tanzania, Uganda, and Zambia.'
Person-to-person transmission has occurred through various means during this outbreak.
There are two types of Monkeypox virus. Historically, clade I has been associated with a higher percentage of people with mpox developing severe illness or dying, compared to clade II. The global outbreak of clade II began in May 2022.
The CDC writes, 'Mpox vaccination is recommended for people who anticipate the following sexual activities during travel to countries with ongoing person-to-person transmission of clade I mpox.'
In the United States, the Bavarian Nordic JYNNEOS® (MVA-BN®, IMVAMUNE®, IMVANEX®) two-dose vaccine is commercially offered at various travel clinics and pharmacies in April 2025.
Those eligible for mpox vaccination should get two doses of JYNNEOS at least 28 days apart, before visiting an mpox outbreak area.

Since the clade II mpox outbreak began about three years ago, the U.S. Centers for Disease Control and Prevention (CDC) has issued Travel Health Advisories based on the type of virus.
Historically, clade I have been associated with a higher percentage of people with mpox developing severe illness or dying, compared to clade II.
On February 10, 2025, the CDC reissued a Level 2 Practice Enhanced Precautions advisory regarding the clade I mpox outbreaks in eight Central and Eastern African countries.
The CDC wrote, 'There is an ongoing person-to-person transmission of mpox in Burundi, Central African Republic, Democratic Republic of the Congo, Kenya, the Republic of the Congo, Rwanda, Uganda, and Zambia.'
In the United States, the New Hampshire Department of Health and Human Services reported the third clade 1b case in the past four months. This mpox patient recently traveled to Eastern Africa.
During these mpox outbreaks, person-to-person transmission has occurred through various means, including sexual contact, day-to-day household contact, and within the healthcare setting. Transmission has also occurred from contact with certain live or dead wild animals.
Mpox is a disease caused by infection with the Monkeypox virus. Symptoms often include fever, rash, headache, muscle aches, and swollen lymph nodes, although fever is not always present.
The CDC says If you are sick and could have mpox, follow isolation and infection control measures at home and during travel.
Mpox vaccination is recommended by the CDC for certain people visiting at-risk areas.
In the U.S. and many countries, mpox vaccines (JYNNEOS®, MVA-BN®) are commercially available in 2025.
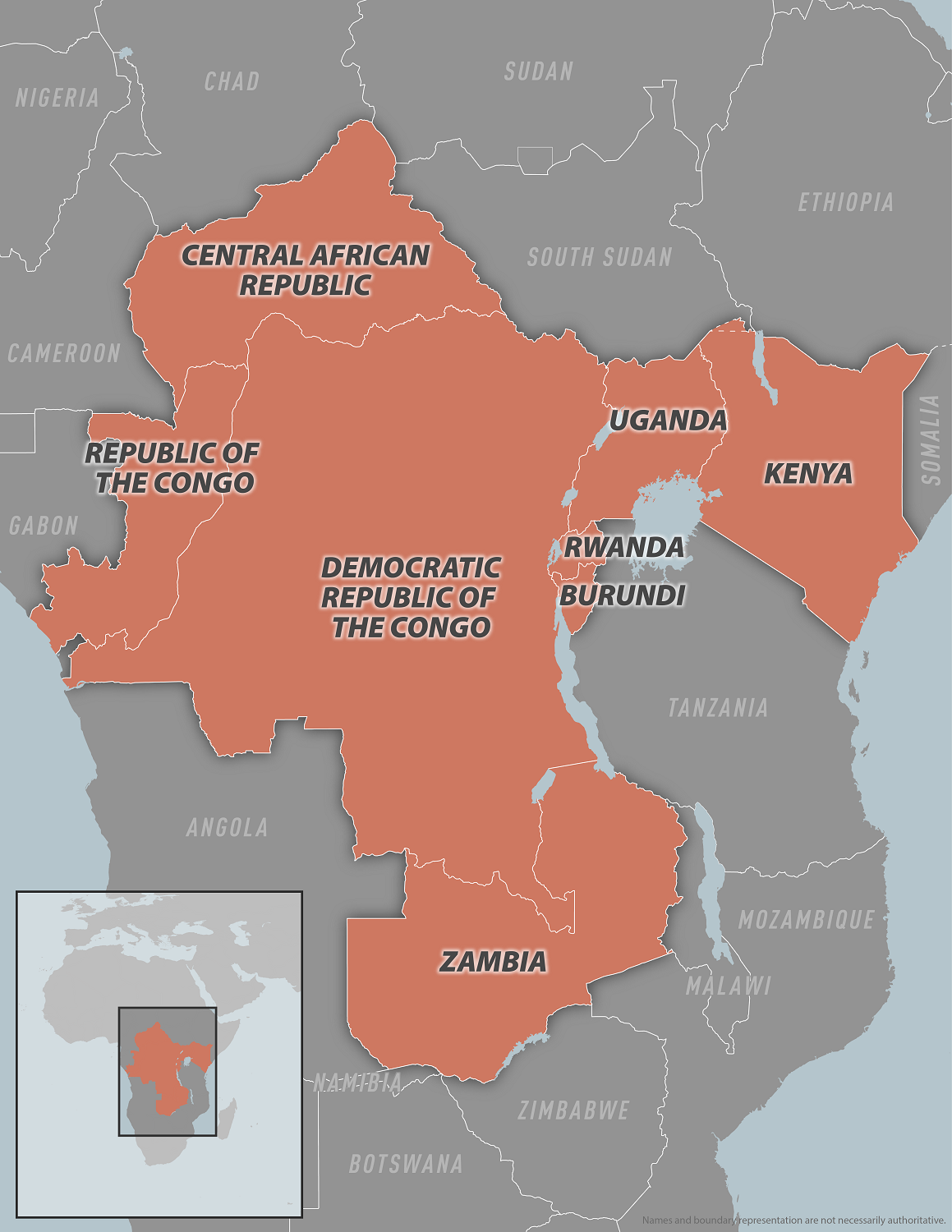
The World Health Organization published its 46th situation report for the multi-country outbreak of monkeypox virus, including reports of new travel-related mpox cases due to clade Ib MPXV.
As of January 28, 2025, the WHO confirmed new travel-related clade Ib MPXV cases had been detected in countries that had already detected travel-related cases, including China, Germany, Thailand, Great Britain and Northern Ireland, and the United States. Azerbaijan has reported its first case during this outbreak.
The outbreak of clade Ib continues predominantly in the Democratic Republic of the Congo, Burundi, and Uganda.
Outside Africa, 11 countries have detected clade Ib MPXV.
In the U. S., since May 2022, most reported mpox cases are clade II.
Additionally, mpox vaccinations are commercially available at clinics and pharmacies in the U.S.

According to the World Health Organization (WHO), the clade Ib monkeypox virus (MPXV) outbreak began in September 2023 and continues predominantly in the Democratic Republic of the Congo, Burundi, and Uganda, with travel-related cases identified in other countries.
In Africa, from January 2024 to January 5, 2025, 14,700 confirmed mpox cases, including 66 deaths (CFR – 0.4%), have been reported by 20 countries. And continues to meet the WHO criteria for a public health emergency of international concern.
As of January 14, 2025, the ECDC reported eleven individuals with MPXV clade I in the EU/EEA since August 2024.
One case was reported by Sweden in August 2024, seven by Germany (one in October, five in December 2024, and one in January 2025), two cases by Belgium in December 2024, and one case by France in January 2025
The WHO says two virus types cause mpox, clade I and II. Both types spread the same way and can be prevented using the same methods, including vaccination.
Most mpox outbreaks in other areas are due to clade IIb MPXV, a continuation of the multi-country outbreak that began in May 2022.
In the United States, the CDC assessed on January 10, 2025, the overall risk to the population(s) posed by the clade I mpox outbreak as low. And clade II mpox is still circulating at low levels.
Various mpox vaccines continue to be available in impacted countries.
Note: Updated on Jan. 14, 2025, to include ECDC data.

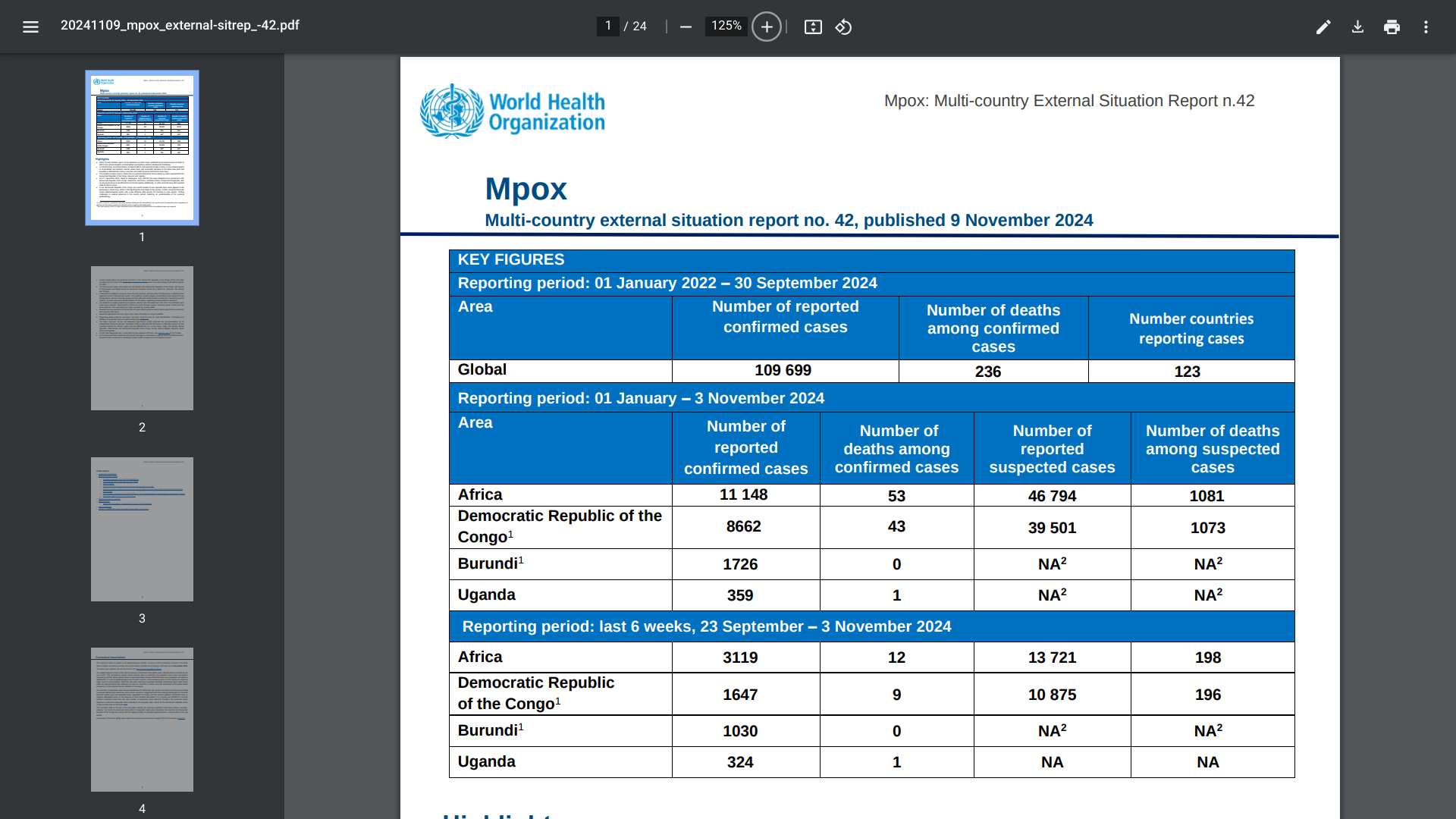
The World Health Organization (WHO) today published its 42nd situation report for the multi-country outbreak of mpox, which provides an update on the epidemiological situation of mpox in the WHO African Region and countries in the WHO Eastern Mediterranean Region.
The number of mpox cases in Africa is generally rising, driven mainly by cases reported from the Democratic Republic of the Congo (DRC).
As of November 11, 2024, the WHO confirmed Clade Ib monkeypox virus (MPXV) had been detected in six provinces in the DRC: South Kivu, North Kivu, Kinshasa, Kasai, Tshopo, and Tanganyika. Additionally, 11 other African countries have also reported clade Ib MPXV cases.
Since the last WHO situation report, three countries outside of Africa have confirmed travel-related cases of clade Ib MPXV,
For the first time, local transmission of clade Ib MPXV in the United Kingdom of Great Britain and Northern Ireland, where three (all) household members of the initial case (who had traveled to affected East African countries with clade Ib) were confirmed to have mpox.
Most impacted countries offer access to Bavarian Nordic's JYNNEOS® (MVA-BN®) vaccine to prevent mpox infections. The first round of mpox vaccination has concluded in the DRC, with around 51 500 people vaccinated across six provinces.
Furthermore, the clade 2 outbreak that began in May 2022 continues in numerous countries, including the United States.
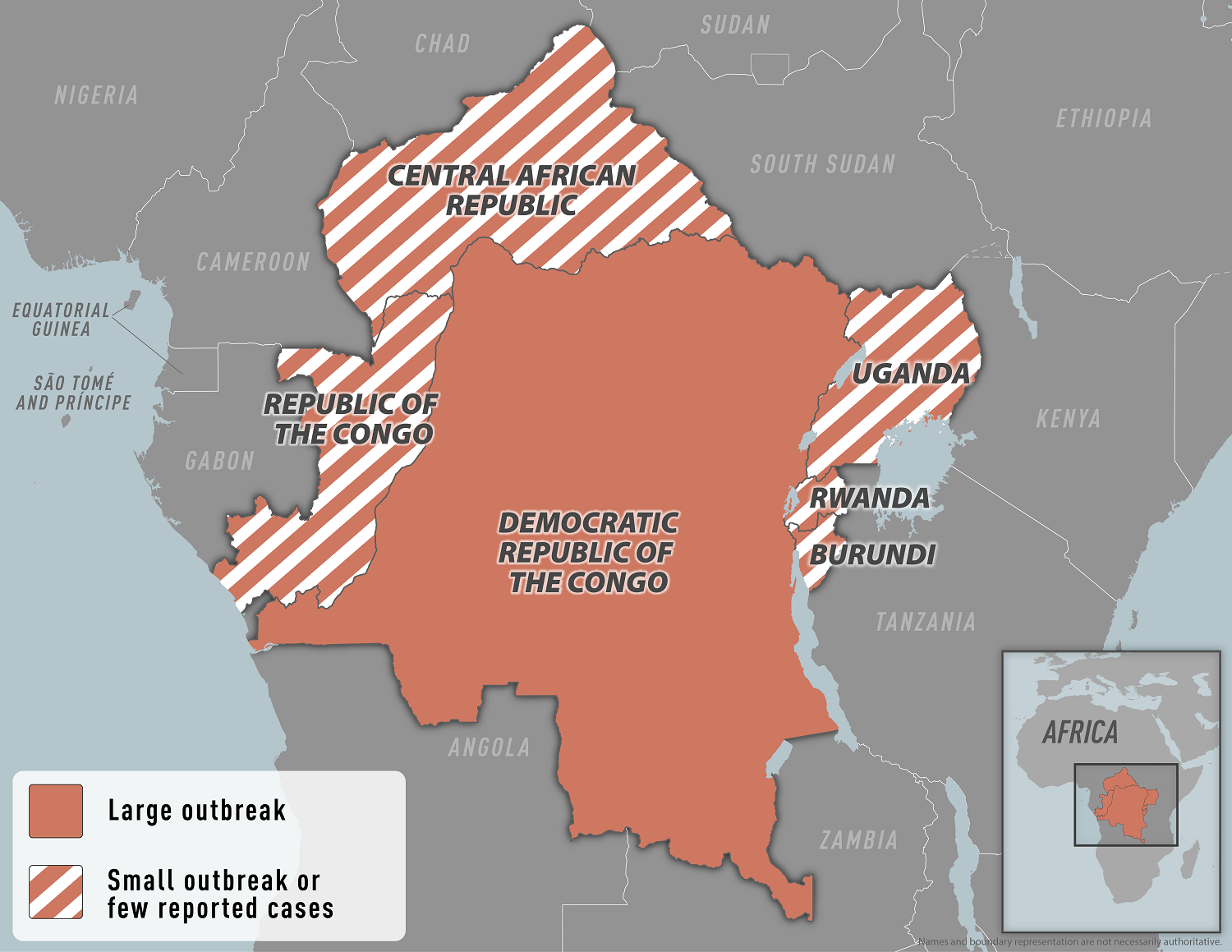
The European CDC recently reported there have been no significant changes in the global circulation of monkeypox virus (MPXV) clade I and clade II during the past week.
In 2024, over 34,000 confirmed and suspected mpox cases due to MPXV clade I and clade II, including over 850 deaths, have been reported from Africa.
To slow the spreading of clade I, the Democratic Republic of the Congo (DRC) announced today vaccination efforts to halt the spread of mpox disease.
The DRC received 265,000 doses of the MVA-BN (Bavarian Nordic A/S, JYNNEOS®) vaccine donated by the European CCommission'sHealth Emergency Preparedness and Response Authority, Gavi, the Vaccine Alliance, and the United States Government.
Vaccinations will be launched in the eastern North Kivu province on October 5, 2024, and will prioritize health workers and frontline responders, contacts of confirmed cases, contacts of those contacts, and other at-risk groups.
Subsequently, the vaccination will be rolled out in eleven of the most affected health zones in Equateur, North Kivu, Sankuru, South Kivu, Sud-Ubangi, and Tshopo provinces.
With a population of about 100 million and assuming two doses per person, 130,000, or 1% of the people in the DRC, can be better protected from mpox.
"The rollout of the vaccine marks an important step in limiting the spread of the virus and ensuring the safety of families and communities,” said Dr. Matshidiso Moeti, WHO Regional Director for Africa, in a WHO press release.
Person-to-person transmission of the MPXV has occurred during this outbreak, including through sexual contact, day-to-day household contact, and within the healthcare setting.
Mpox vaccination is now recommended for most people visiting outbreak areas, says the ECDC. There are now several Mpox vaccines available worldwide.
The U.S. Centers for Disease Control and Prevention (CDC) today issued Health Alert Network Health Update (CDCHAN-00516) to provide additional information about the ongoing outbreak of clade I monkeypox virus (MPXV), the virus that causes mpox.
As of September 23, 2024, no cases of clade I mpox have been identified in the U.S.
This mpox strain is more severe than the clade 2 strain circulating in the U.S. since May 2022.
The CDC urges travelers to vaccinate against mpox if they are heading to Eastern and Central African countries where clade 1 MPXV has been spreading.
Furthermore, healthcare providers and travel vaccine experts should recommend vaccination to those whose activities place them at risk.
This is essential advice since clade 1 mpox cases have recently been confirmed in international travelers.
For example, The Mint reported a man from Malappuram district in Kerala, India, has been detected with Mpox clad I. The patient had returned from the United Arab Emirates.
According to the CDC, two doses of JYNNEOS® (MVA-BN®, IMVAMUNE®, IMVANEX®) should be given at least six weeks before traveling abroad. This U.S. FDA-approved vaccine is available at clinics and pharmacies in the U.S.

- 1 of 20
- next ›