Search API
Carnival Cruise Line announced on January 28, 2022, it is pausing selected Australian sailings beginning mid-March 2020 through to the end of May 2022.
Carnival's statement indicates the disruption in service of Carnival Splendor and Carnival Spirit is related to the ongoing COVID-19 pandemic in Australia.
The delayed sailings for Carnival Splendor are through May 31, 2022, and for Carnival Spirit through May 29, 2022.
'On behalf of all of us at Carnival Cruise Line, we extend our sincere apologies for the disruption to your holiday plans and trust you understand this decision was made with everyone's best interest at heart.'
'Since we'd love nothing more than to welcome you back just as soon as we resume operations, we're pleased to present the following Enhanced Value Option for those booked on affected cruises.' says Carnival's updated website.
In contrast with Australis, the U.S. cruise industry appears to be very active.
On January 21, 2022, Carnival Cruise Line confirmed that it would be the largest cruise line to use the VeriFLY solution for its operations from U.S. homeports, with a pilot program launching with Mardi Gras at Port Canaveral.
Once the pilot at Port Canaveral is complete, Carnival intends to move quickly to implement the solution across its fleet.
VeriFLY by Daon is now widely used in the airline industry.
It will allow Carnival guests to upload their proof of vaccination and testing information to be verified in advance of the sailing, resulting in a streamlined embarkation experience at the terminal.
And on January 13, 2022, Carnival restarted operations, with Carnival Sunshine returning to its year-round schedule from Charleston, SC.
To date, Carnival has 19 of its 22 U.S.-based ships in guest operation, sailing from eight homeports on the Atlantic, Gulf, and West coasts.
Carnival Cruise Line is a global cruise line, with some five million guests traveling every year, and whose fleet will total 25 ships in 2022. Carnival sails to various destinations worldwide, including the Caribbean, Mexican Riviera, Alaska, South Pacific, Australia, New Zealand, and more.

The U.S. Department of State issued a high-level advisory on January 26, 2022, saying, 'Do not travel to the United Arab Emirates (UAE) due to COVID-19 and civil unrest.'
And the Centers for Disease Control and Prevention (CDC) issued a Level 4 Travel Health Notice due to COVID-19 on January 24, 2022, indicating the risk of contracting COVID-19 has increased.
The CDC says 'before planning any international travel, please review the specific recommendations for vaccinated and unvaccinated travelers.
And if you must travel to the UAE, ensure you are vaccinated and up-to-date with your COVID-19 vaccines before traveling.
The CDC clarified being 'up-to-date' indicates a person has received all recommended COVID-19 vaccines, including any booster dose(s) when eligible.
Furthermore, the State Department stated 'the possibility of attacks affecting U.S. citizens and interests in the Gulf and Arabian Peninsula remains an ongoing, serious concern. Groups operating in Yemen have stated an intent to attack the UAE.'
And due to risks to civil aviation operating within the Persian Gulf and the Gulf of Oman region, including the UAE, the Federal Aviation Administration has issued an Advisory Notice to Airmen and/or a Special Federal Aviation Regulation.
For more information, U.S. citizens should consult the Federal Aviation Administration's Prohibitions, Restrictions, and Notices.
And Americans seeking assistance should visit the U.S. Embassy's webpage for more information on restrictions and conditions in the UAE. The Embassy is located at Plot 38, Sector W59-02, Street No. 4., Abu Dhabi, UAE.
The Belgium-based European Council (EC) announced on January 25, 2022, it adopted a recommendation on a coordinated approach 'facilitating safe free movement during the COVID-19 pandemic.'
The recommendation will enter into force on February 1, 2022.
This EC recommendation responds to the significant increase in COVID-19 vaccine uptake and the rapid roll-out of the EU digital COVID certificate.
As of January 28, 2022, Europe reported about 70% of its population were vaccinated with a 'primary course.'
And, under the new EC recommendation, COVID-19 measures should be applied, taking into account the status of the person instead of the situation at a regional level, with the exception of areas where the virus is circulating at very high levels.
This means that a traveler's COVID-19 vaccination, test, or recovery status should be the key determinant, as evidenced by a valid EU digital COVID certificate.
And could require people to receive their booster shots to keep their COVID-19 passe valid.
'A person-based approach will substantially simplify the applicable rules and will provide additional clarity and predictability to travelers.'
However, persons who do not have an EU digital COVID certificate could be required to undergo a test before or no later than 24 hours after arrival. And travelers with an essential function or need, cross-border commuters, and children under the age of 12 should be exempt from this requirement.
Located in Brussels, the EC consists of the heads of state or government of the EU's member states, together with its President and the European Commission President. It defines the EU's general political direction and priorities. However, the Council recommendation is not legally binding instruments.

The World Health Organization (WHO) reported that global influenza levels remained low on January 24, 2022, and appeared to be dissipating.
The WHO's Influenza Update N° 411 says, 'The current influenza surveillance data should be interpreted with caution as the ongoing COVID-19 pandemic has influenced to varying extents global health-seeking behaviors.'
The National Influenza Centres and other national influenza laboratories recently reported data from 99 countries, areas, or territories. These laboratories tested more than 317,198 specimens and identified 16,862 positives for influenza viruses.
This data reflects a .05% infectious rate.
From a regional perspective, the WHO reported:
- In North America, influenza detections remained low compared to similar periods in past seasons (except 2020-2021). In addition, RSV activity continued to decrease in the USA and Canada.
- Some influenza activity was reported in the Caribbean and Central American countries with influenza A(H3N2) predominating.
- Europe, influenza activity appeared to decrease.
- In East Asia, influenza activity continued on an increasing trend in China, while influenza illness indicators and activity remained low in the rest of the subregion.
- Western Asia and Northern Africa, influenza transmission has been reported in some countries.
- In Tropical Africa, overall influenza activity continued on a decreasing trend.
- South America, influenza A(H3N2) detections remained elevated. In addition, severe acute respiratory infection levels were above the epidemic threshold in some countries.
- South-East Asia, sporadic influenza detections were reported in the Philippines.
And in the temperate zones of the Southern Hemisphere, influenza activity remained low overall, although increased detections of influenza A(H3N2) were reported in some countries in temperate South America.
The WHO's Global Influenza Programme monitors influenza activity worldwide and publishes an update every two weeks.
Separately, the U.S. CDC reported on January 21, 2022, 'influenza activity declined slightly again this week. While influenza activity is difficult to predict, it is expected to continue for several more weeks.'
The percentage of outpatient visits due to respiratory illness decreased nationally again this week but remained above baseline. And the number of hospital admissions reported to HHS Protect declined slightly this week.
Influenza contributes to respiratory illness levels, but other respiratory viruses are also circulating. Therefore, the relative contribution of influenza varies by location.
'There's still time to get vaccinated,' added the CDC.
'An annual flu vaccine is the best way to protect against flu and potentially severe complications.
The CDC recommends everyone six months and older get a flu vaccine. Flu vaccines are available at many locations, including pharmacies and health departments. Visit www.vaccines.gov to find a flu vaccine near you.

The U.S. Embassy in Manila announced today all airline passengers heading to the United States ages two years and older, regardless of COVID-19 vaccination status or citizenship, must provide a negative COVID-19 viral test taken within one calendar day before travel.
Alternatively, travelers to the U.S. may provide documentation from a licensed healthcare provider of having recovered from COVID-19 in the 90 days preceding travel.
And please visit the U.S. CDC webpage for more information about exceptions and the requirement for proof of a negative COVID-19 test or recovery from COVID-19 for all air passengers arriving in the U.S.
Additionally, the CDC has determined that for entry into the U.S., acceptable COVID-19 vaccines include those U.S. FDA approved/authorized and vaccines Listed by the World Health Organization.
Furthermore, to ensure the health and safety of our clients and our staff, the U.S. Embassy in Manila has rescheduled all non-emergency appointments for U.S. passports and Consular Reports of Birth Abroad through January 31, 2022.
For more information, see the Embassy's Health Alert issued on January 19, 2022.
And according to the Philippine Inter-Agency Task Force for the Management of Emerging Infectious Diseases, several locations are currently under Alert Level 3 and 4 until January 31, 2022.
The Philippine government has also mandated everyone must wear face masks in public places.
In addition, the use of face shields is mandatory in medical and quarantine facilities, as well as during Alert Level 5, Enhanced Community Quarantine, and granular lockdowns.
Moreover, a recent presidential order empowers local officials to restrict the movements of unvaccinated individuals in the Philippines.
For more information on applying for an exception through the U.S. Embassy in Manila, visit this webpage.
The U.S. Embassy in Manila is located at 1201 Roxas Boulevard, Manila, Philippines 1000. Phone: (632) 5301-2000.
Note: before the COVID-19 pandemic, the number of visitors to the U.S. from the Philippines exceeded 310,000 annually.
Cancer Research UK announced on January 24, 2022, it intends to invest £100 million over five years into Cancer Research UK Centres in Cambridge, City of London, Convergence Science, Manchester, Newcastle, Oxford, and Scotland.
'The seven funded Centres are central to our ambition to beat cancer, providing a key link between laboratory research and patient-focused studies,' commented Michelle Mitchell, CEO, Cancer Research UK.
'We award Centre status to locations that perform the highest quality cancer research.'
'Our funding supports essential research infrastructure including technical staff, equipment, pump-priming grants, and training, to further develop the breadth and depth of research at each of these Centres.'
'Our Centres bring together a network of research teams from local universities, NHS hospitals, and other research organizations.'
'They seek to understand the impact and efficacy of treatments to initiate new research ideas and programs, translating cutting-edge discoveries from the laboratory into direct benefits for patients.'
'Standards were particularly high, in part, because our investment of £100 million is a reduction of around a third compared to the last funding round in 2017.'
'While the COVID-19 pandemic has required us to reshape our research model and make tough decisions about the research we fund, this investment continues to provide vital infrastructure for the bench to bedside research, as well as creating opportunities for the next generation of scientists.'

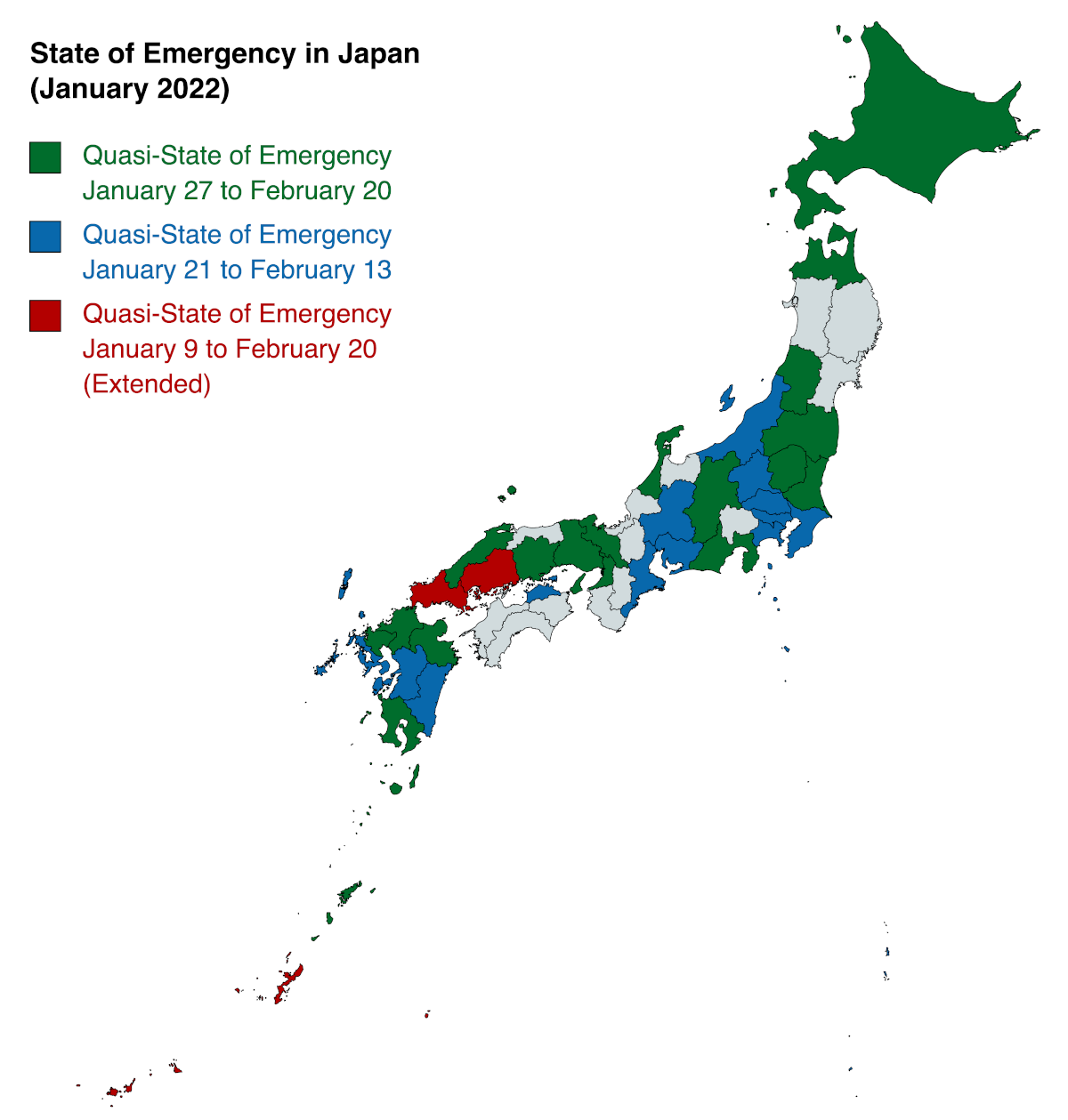
In response to a rapid rise in the number of COVID-19 cases, the U.S. Embassy in Japan announced today the Japanese government had extended the quasi-state of emergency for an additional 18 prefectures that will remain in effect through February 20, 2022.
These measures will prevent the spread of COVID-19 in Hokkaido, Aomori, Yamagata, Fukushima, Tochigi, Ibaraki, Nagano, Shizuoka, Ishikawa, Osaka, Kyoto, Hyogo, Okayama, Shimane, Fukuoka, Oita, Saga, and Kagoshima.
Additionally, the previously declared quasi-state of emergency for Tokyo, Kanagawa, Saitama, Chiba, Gunma, Niigata, Kagawa, Aichi, Mie, Gifu, Nagasaki, Kumamoto, and Miyazaki prefectures will remain in effect through February 13.
And Yamaguchi, Hiroshima, and Okinawa prefectures have been extended and will remain in place until February 20.
Furthermore, the number of prefectures in Japan under a quasi-state of emergency may change with little or no notice.
The quasi-state of emergency will allow prefectural governments to impose targeted measures to slow the spread of COVID-19.
These measures may include, but are not limited to, shortened business hours for eateries and other establishments, restrictions on the sale of alcohol in restaurants and bars, and/or limits on the number of customers in enclosed spaces.
U.S. citizens in Japan are urged to follow all national and prefectural COVID-related guidance to protect their health.
Furthermore, U.S. citizens and foreign nationals who are fully vaccinated should travel with proof of their COVID-19 vaccination status to provide to their airline before departure to the U.S.
Proof of vaccination should be a paper or digital record issued by an official source. It should include the COVID-19 vaccine product and date(s) of administration for all doses received.
And the U.S. CDC has determined that for purposes of travel to the U.S., COVID-19 vaccines accepted will include FDA approved/authorized and World Health Organization Listed vaccines.
See this link for FAQ information.
U.S. citizens needing assistance should contact the U.S. Embassy Tokyo; Telephone: 03-3224-5000; after-hours: 03-3224-5000; Email: TokyoACS@state.gov; https://jp.usembassy.gov/services/.
Japan-based Chugai Pharmaceutical Co., Ltd. announced on January 21, 2022, that it obtained regulatory approval from the Ministry of Health, Labour, and Welfare for the Actemra® humanized anti-human IL-6 receptor monoclonal antibody (mAbs).
Actemra has already obtained regulatory approval for various indications in more than 110 countries.
Japan's approval came one month after the application for the additional indication of Actemra Intravenous Infusion 80 mg, 200 mg, and 400 mg for the additional indication of the treatment of SARS-CoV-2 pneumonia, limited to patients requiring oxygen intervention.
"Clinical studies demonstrated that Actemra reduced the mortality rate in patients with SARS-CoV-2 infection," Chugai's President and CEO, Dr. Osamu Okuda, commented in a related press statement.
"We hope that Actemra will play a role for the better prognosis of patients with severe, potentially life-threatening pneumonia."
This COVID-19 treatment approval is based on the results from clinical studies evaluating Actemra in hospitalized patients, including an investigator-initiated, randomized, open-label, platform overseas study (RECOVERY study), three placebo-controlled, randomized, double-blind, multicenter, global phase III studies conducted by Roche (COVACTA study, EMPACTA study, REMDACTA study), and a single-arm, multicenter phase III study in Japan (J-COVACTA study).
Actemra has been approved in the European Union, authorized for emergency use in the United States, and recommended by the World Health Organization to treat COVID-19.
Actemra is designed to block the activity of IL-6, a type of inflammatory cytokine. First launched in June 2005, the intravenous injection is approved for six indications in Japan: Castleman's disease, rheumatoid arthritis, systemic juvenile idiopathic arthritis, polyarticular juvenile idiopathic arthritis, cytokine release syndrome induced by tumor-specific T cell infusion therapy, and adult Still's disease.
In addition, Actemra subcutaneous injection is approved for three indications in Japan: rheumatoid arthritis, Takayasu arteritis, giant cell arteritis.

Florida-based AIM ImmunoTech Inc. today announced the publication of positive data from a Phase 1/2 clinical study of intraperitoneal chemo-immunotherapy in advanced, recurrent ovarian cancer. The chemokine-modulating IP chemo-immunotherapy combination was demonstrated to be well-tolerated and associated with interferon-stimulated gene changes that favor cytotoxic T lymphocytes chemoattraction and function.
"These results represent an important extension of prior studies using human tumor explants that showed Ampligen's potentially important role as a TLR3 agonist acting synergistically with high-dose IFNα and celecoxib to selectively enhance Teff cell-attractants while suppressing Treg-attractants in the tumor microenvironment with a concomitant increase in the Teff/Treg ratio," stated David Strayer, MD, Chief Medical Officer, Chief Scientific Officer of the Company, and Board Certified in Medical Oncology, in a press release issued on January 24, 2022.
"This current study shows that similar findings are seen combining Ampligen with chemokine-targeting and chemo-immunotherapy in patients being treated for recurrent ovarian cancer."
"The importance of boosting the Teff/Treg ratio in the tumor microenvironment is that it is associated with the conversion of 'cold' tumors into 'hot' tumors, which have an increased sensitivity to chemo-immunotherapy and an improved chance of showing tumor regression."
"This, of course, creates the potential for a positive survival advantage,"
A phase 2 clinical study will be conducted to evaluate the immunologic and clinical efficacy of tumor loaded αDC1 vaccine in conjunction with the cisplatin/chemokine modulatory combination regimen.
The Phase 1/2 study being conducted by the University of Pittsburgh School of Medicine is designed to evaluate the immunologic and potential clinical effectiveness of intensive loco-regional sequential intraperitoneal (IP) cisplatin (IPC) with intravenous (iv) paclitaxel followed by peritoneal infusion of a chemokine modulatory (CKM) regimen composed of a cocktail of IP rintatolimod and interferon-alpha (IFNα) for patients with advanced-stage ovarian cancer (III-IV) at primary neoadjuvant setting. AIM ImmunoTech provided rintatolimod (Ampligen®, a dsRNA acting as TLR3 agonist) for the Phase 1/2 study.
The manuscript titled, "Phase I trial combining chemokine-targeting with loco-regional chemo-immunotherapy for recurrent, platinum-sensitive ovarian cancer shows induction of CXCR3 ligands and markers of type 1 immunity1" was published in the American Association for Cancer Research publication, Clinical Cancer Research, on January 19, 2022.
The U.S. CDC says 'ovarian cancer is the second most common gynecologic cancer in the United States. Ovarian cancer causes more deaths than any other cancer of the female reproductive system.
And the U.S. Cancer Statistics Data Visualizations Tool makes it easy to explore the latest cancer data from United States Cancer Statistics.
AIM ImmunoTech Inc. is an immuno-pharma company located in Ocala, FL, focused on the research and development of therapeutics to treat multiple types of cancers, immune disorders, and viral diseases, including COVID-19, the disease caused by the SARS-CoV-2 virus.

