Search API
UNICEF recently announced an agreement to secure supplies of the R21/Matrix-M™ malaria vaccine. The long-term agreement signed with Serum Life Sciences for 2024 – 2028 is conditional on vaccine pre-qualification from the World Health Organization.
Under the four-year agreement, UNICEF expects to begin delivering the R21/Matrix-M vaccine in mid-2024.
This procurement agreement will help boost the global malaria vaccine supply and accelerate equitable access for children and families.
“It is heartbreaking and unacceptable that almost half a million children die of malaria every year. This agreement is a critical step towards protecting more children from this deadly disease,” said the Director of UNICEF Supply Division, Leila Pakkala, in a press release on October 12, 2023.
The R21/Matrix-M vaccine includes Novavax AB proprietary saponin-based Matrix-M adjuvant and is licensed to and manufactured by the Serum Institute of India Private Ltd.
The R21 was created by the University of Oxford Jenner Institute.
The R21/Matrix-M and RTS,S (Mosquirix™) malaria vaccines are the first vaccines developed against a parasitic disease. Both act against Plasmodium falciparum, the deadliest malaria parasite globally and the most prevalent in Africa.
UNICEF is the world’s largest single vaccine buyer, procuring more than two billion doses of vaccines annually for routine child immunization and outbreak response on behalf of nearly 100 countries.
Although malaria was eliminated in the United States in the mid-1950s, approximately 2,000 malaria cases are imported into the country from regions with endemic disease transmission each year, according to the U.S. CDC.
Anopheles mosquito species can transmit malaria. However, locally acquired mosquito-transmitted cases have not been identified in the U.S. since 2003.
In mid-2023, eight malaria cases (Plasmodium vivax) were identified in Florida and Texas. In both states, the locally-acquired cases occurred in the vicinity of an imported malaria case.
The U.S. reports about 1,900 travel-related malaria cases annually.
The CDC has issued various malaria outbreak alerts for endemic countries, including Costa Rica.

With the respiratory season arriving in the United States, a seldom-mentioned disease surpassed both COVID-19 and Influenza mortality last week.
According to the U.S. National Center for Health Statistics Mortality Surveillance reporting on October 13, 2023, there were 1,226 pneumonia deaths last week, exceeding 549 related to COVID-19 and 10 for influenza.
And no influenza-associated pediatric deaths occurred during the first week of the 2023-2024 flu season. Last flu season, there were 178 influenza-associated pediatric deaths.
Furthermore, the U.S. CDC reported outpatient respiratory illness was below baseline in all 10 HHS regions, and flu-related hospital admissions remained low nationally.
The good news is ample supplies of vaccines are available for the 2023-2024 respiratory season in the U.S. There are over 110 million flu shots and updated COVID-19 vaccines available today.
Regarding pneumonia, which is an infection of the lungs that can cause mild to severe illness in people of all ages, the CDC says various immunizations can help prevent disease by some of the bacteria and viruses that can cause pneumonia.
These immunizations include but are not limited to COVID-19, Influenza, Measles, Pertussis, Pneumococcal, Respiratory syncytial virus, and Varicella.
These immunizations are safe, but side effects can occur, most of which are mild, and dissipate on their own within a few days, says the CDC. These vaccines are generally available at health clinics and pharmacies in the U.S.

Ultimovacs ASA today announced encouraging overall survival (OS) data from cohort 1 in the UV1-103 Phase I clinical trial in malignant melanoma.
Among the patients in cohort 1 who were alive at the 3-year follow-up, no further deaths have been reported, reaffirming an encouraging trend of durable overall survival benefit from UV1 vaccination.
The 4-year survival across both cohorts is expected to be announced in Q2 2024.
Ultimovacs has previously reported data showing a complete response rate in the UV1-103 study of 33% (complete disappearance of tumors) and an objective response rate of 57% (complete or partial disappearance of tumors).
Biomarker analyses reported in October 2022 showed robust clinical responses in patients treated with the combination of UV1 and pembrolizumab, regardless of patients' PD-L1 status. The safety profile of UV1 in combination with pembrolizumab is comparable to that of pembrolizumab alone.
UV1 is a peptide-based vaccine inducing a specific T-cell response against the universal cancer antigen telomerase.
"We are very encouraged to report a durable and long-term overall survival rate at the 4-year follow-up in the UV1-103 study. The data further strengthen the previously reported results from the study, including good safety for UV1 and the high number of complete responses in patients with metastatic malignant melanoma where surgery is not an option," said Jens Bjørheim, Chief Medical Officer at Ultimovacs, in a press release on October 12, 2023.
"The UV1-103 study treats the same patient population as our Phase II study INITIUM. As we await data from the first three randomized UV1 Phase II trials in the near-term, we are increasingly optimistic about UV1's potential to benefit cancer patients."
Ultimovacs is investigating UV1 in malignant melanoma in its randomized Phase II INITIUM trial of UV1 in combination with ipilimumab and nivolumab.
The top-line results will be disclosed after cancer progression has been verified in 70 patients, which has not yet occurred due to patients taking longer than estimated to experience cancer progression. The study's outcome is expected to be announced in the first half of 2024.

A glioblastoma vaccine therapy candidate has been granted Fast Track Designation (FTD) by the United States Food and Drug Administration (FDA).
Announced on October 12, 2023, MimiVax Inc.'s SurVaxM vaccine (immunotherapy) is being studied to treat newly diagnosed glioblastoma (nGBM).
Glioblastoma is a rare disease with great unmet medical need.
SurVaxM is a first-in-class, patented peptide mimic immunogen that targets survivin, a cell-survival protein present in 95% of glioblastomas and in many other cancers.
SurVaxM stimulates a patient’s immune response to control tumor growth and prevents disease recurrence. Because survivin is present in most cancers, SurVaxM could potentially have applicability in other cancers.
"The receipt of FTD affirms the importance of new clinical developments of novel therapies to improve the treatment and outcomes for patients with newly diagnosed glioblastoma," said Michael Ciesielski, CEO of MimiVax, in a press release.
"This designation is a key component in our journey to help patients with glioblastoma to live longer."
A randomized, blinded, placebo-controlled Phase 2b clinical trial of SurVaxM for nGBM (SURVIVE) [NCT05163080] is now recruiting at 11 cancer centers across the U.S.
Previously, positive Final Data from the previous Phase 2a Study of SurVaxM for nGBM, published in the Journal of Clinical Oncology, found that 51% of patients receiving SurVaxM survived at least two years, and 41% survived at least three years.
The median Overall Survival of 25.9 months with nGBM in this study is considerably higher than expected with standard therapy alone.

During the 2023 Grand Challenges Annual Meeting, Bill Gates, Co-chair of the Bill & Melinda Gates Foundation, announced new $40 million investments to advance access to mRNA research and vaccine manufacturing technology that will support low- and middle-income countries (LMICs) capacity to develop high-quality, lifesaving vaccines.
Announced on October 9, 2023, this new funding builds on the Gates Foundation's previous $55 million investment in mRNA manufacturing technology.
"Expanding the availability of affordable, high-quality vaccines that meet the needs of local communities is one of the best ways to improve global health outcomes and reduce preventable deaths," commented Trevor Mundel, president of the Gates Foundation's Global Health Division, in a press release.
"By lowering barriers to access for LMICs, we can help ensure more people around the world benefit from lifesaving health innovation."
The foundation's funding advances access to Quantoom Biosciences' low-cost mRNA research and manufacturing platform, developed with an early-research Grand Challenges grant made to its parent company, Univercells.
The Institut Pasteur de Dakar and Biovac, research institutes with vaccine manufacturing experience based in Senegal and South Africa, respectively, will receive US$5 million each to acquire the technology and will be able to use it to develop locally relevant vaccines.
To further advance the technology and lower costs for commercialization, the foundation will also provide US$20 million to Quantoom Biosciences, ensuring LMICs can benefit from the next-generation mRNA health tools.
The Gates Foundation will grant another US$10 million to other LMIC vaccine manufacturers to be named.

LimmaTech Biologics AG announced today the closing of a USD 37 million Series A financing round that will empower its proprietary technology platform and accelerate its preclinical and clinical vaccine candidates against increasingly dangerous bacterial infections, including programs addressing shigellosis and gonorrhea.
Antimicrobial Resistance is responsible for approximately 5 million deaths annually. Infections that were once easily treatable have now become difficult, if not impossible, to cure.
As a leading example of this threat to global health, half of the approximately 700,000 annual gonorrhea infections in the U.S. are already resistant to antibiotics, and there is a real threat of gonorrhea soon becoming untreatable.
While there are no gonorrhea vaccines available, off-label vaccines and treatments are in use.
Later-stage clinical development efforts will focus on the company's Shigella vaccine program, which LimmaTech developed with GSK. The company expects to announce preliminary results from the Shigella program's ongoing Phase 2 clinical trial in the second half of 2023.
Shigella cause an estimated 450,000 infections in the U.S. each year.
According to the U.S. CDC, people can get a Shigella infection (shigellosis) after putting something in their mouth or swallowing something that has come into contact with the stool of someone with a Shigella infection.
"Within the next decade, multiple bacterial infections will become untreatable due to antimicrobial Resistance, which is already a significant burden on global health. By advancing our innovative technology platform, LimmaTech has the potential to simultaneously provide vaccine-induced protection against bacterial infections, mitigate the increasing risk of antibiotic resistance, and move toward the control of several highly transmissible pathogens," commented Dr. Franz-Werner Haas, CEO of LimmaTech, in a press release on October 9, 2023.
".....With this support and our team of proven experts in bacterial vaccine development and manufacturing, we look forward to providing life-changing vaccines to address a major global medical need."
The Company is conducting a Phase I/II clinical trial in the Republic of Kenya of a 4-valent candidate vaccine to help prevent diarrheal disease caused by the Shigella bacteria in children and infants in low and middle-income regions. The Shigella study is conducted in collaboration with GSK and the Wellcome Trust.
LimmaTech is committed to translating novel scientific concepts into highly effective vaccines that benefit humanity. For more information, please visit www.lmtbio.com.

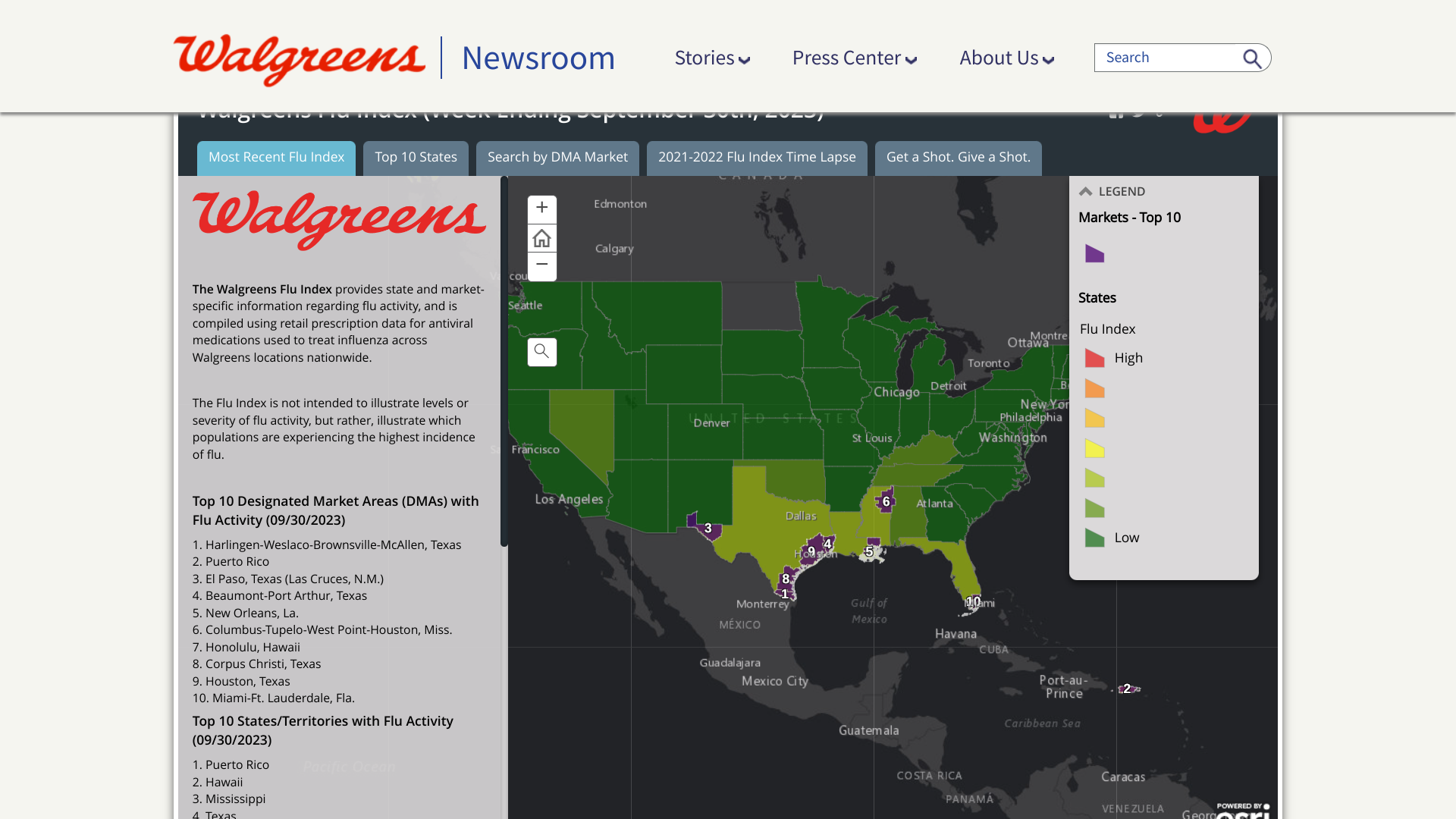
While the United States community is debating where and when the 2023-2024 flu season will arrive, the Walgreens Flu Index® ranks the top markets and states for flu activity, including Puerto Rico.
As of September 30, 2023, cities in the southern U.S. led the Top 10 Designated Market Areas with Flu Activity.
- Harlingen-Weslaco-Brownsville-McAllen, Texas
- Puerto Rico (San Juan)
- El Paso, Texas (Las Cruces, N.M.)
- Beaumont-Port Arthur, Texas
- New Orleans, La.
- Columbus-Tupelo-West Point-Houston, Miss.
- Honolulu, Hawaii
- Corpus Christi, Texas
- Houston, Texas
- Miami-Ft. Lauderdale, Fla.
From a more clinical perspective, the U.S. Centers for Disease Control and Prevention (CDC) today published its week #39 Influenza Surveillance Report.
As of October 6, 2023, seasonal flu rates last week were low nationally, with 444 (1%) positive specimens reported last week.
Additionally, 1,040 patients with laboratory-confirmed influenza were admitted to a hospital, an increased number since the previous CDC report.
And the U.S. National Center for Health Statistics mortality surveillance data distributed on October 5, 2023, confirmed that 11 additional influenza-related deaths were reported last week.
Of the flu-related deaths reported from October 2, 2022, to September 9, 2023, 9,697 (4%) listed influenza.
This data exceeded the average number of influenza-coded deaths (8,530) from 2015-16 through 2019-20.
Among 5,390 hospitalized adults with information on underlying medical conditions, 96.8% had at least one reported underlying medical condition; the most commonly reported were hypertension, cardiovascular disease, metabolic disorder, and obesity.
The CDC's new Director, Mandy K. Cohen, MD, MPH, recently answered questions and offered the most up-to-date information and common-sense solutions so you can protect yourself and your loved ones this respiratory season.
This week's updated flu shot availability news indicated that over 100 million influenza vaccines had been distributed to health clinics and pharmacies in the U.S.