Search API
According to an analysis from the U.S. Centers for Disease Control and Prevention (CDC) and published by NEJM Evidence on September 13, 2024, SIGA Technologies, Inc. mpox treatment tecovirimat (TPOXX®) safety and effectiveness against the monkeypox virus clade 2 virus can't be determined from data.
CDC researchers evaluated data from over 7,100 patients prescribed tecovirimat, a virostatic antiviral drug, from May 29, 2022, through July 10, 2023.
They wrote, 'Although relatively few serious adverse events (SAEs) were reported, because of the passive nature of reporting, we cannot definitively conclude that tecovirimat treatment was always safe. Similar to data from case reports and other published observational studies, our data, in the absence of comparison data from untreated patients, cannot be used to infer clinical effectiveness, or lack thereof, of tecovirimat treatment.'
Overall, 223 SAEs and 40 deaths were reported. Most events were among patients who were severely immunocompromised.
Despite the inclusion of many patients with severe disease for whom the CDC was consulted, outcomes were favorable for most of the treated patients in this cohort.
This analysis did not review the current mpox clade 1 outbreak impacting countries in Africa.

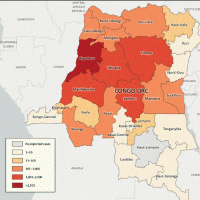
The World Health Organization (WHO) today announced temporary (one-year) recommendations for States Parties experiencing the upsurge of monkeypox virus (MPXV) clade 1 detections, including, but not limited to, the Democratic Republic of the Congo (DRC), Burundi, Kenya, Rwanda, and Uganda.
The upsurge of mpox cases in the DRC in 2024 and its neighboring countries is driven by outbreaks associated with two sub-clades of clade I MPXV: clade Ia and clade Ib.
These WHO recommendations include establishing or strengthening cross-border collaboration arrangements for surveillance and management of suspect mpox cases and providing information to travelers and conveyance operators without resorting to general travel and trade restrictions unnecessarily impacting local, regional, or national economies.
As of August 19, 2024, the WHO Committee considered the event “extraordinary” because of the increase in mpox clade I disease occurrence in the DRC and the emergence of the new MPXV clade Ib.
Clade I mpox was classically described in studies conducted by WHO in the 1980s to have a mortality rate of approximately 10%, with most deaths occurring in children.
MPXV clade Ia is endemic in the DRC. The disease primarily affects children. Data available for 2024 show an aggregated case fatality rate of 3.6%, and the spread is likely sustained through multiple modes of transmission, including person-to-person transmission following zoonotic introduction in a community.
MPXV clade Ib is a new strain of MPXV that emerged in the DRC. It is transmitted between people, presumed via sexual contact, which has been spreading in the eastern part of the country.
Although first characterized in 2024, estimates suggest it emerged around September 2023.
The outbreak associated with clade Ib in the DRC primarily affects adults and is spreading rapidly, sustained largely, but not exclusively, through transmission linked to sexual contact and amplified in networks associated with commercial sex and sex workers.
Furthermore, these African countries are to initiate plans to advance mpox vaccination activities targeting people at high risk of infection. As of August 19, 2024, various reports indicate that (10 million) mpox vaccines are being produced to meet potential outbreak demand.
In early August 2024, the U.S. CDC issued a Level 2 - Practice Enhanced Precautions, Travel Health Advice, recommending various mpox protection tactics, including (JYNNEOS) vaccination.
These new WHO recommendations are intended to be implemented by those States Parties in addition to the current standing recommendations for mpox, which will be extended until August 20, 2025.

The Democratic Republic of the Congo (DRC) is having its largest surge of mpox cases ever recorded, says the U.S. CDC. Since January 2023, the DRC has reported more than 20,000 suspected mpox cases, and about 1,000 deaths have been reported.
To notify international travelers of their potential mpox risk, the CDC reissued a Travel Health Advisory on June 10, 2024.
Mpox is a disease caused by infection with the monkeypox virus. It is divided into two Clades. Clade IIb is responsible for the global outbreak that began in May 2022, while Clade 1 is causing the Mpox outbreak in the DRC.
There have been no cases of the type of mpox spreading in DRC reported in the United States, says the CDC. The risk to the general public in the U.S. from this type of mpox is very low.
In 2023, the CDC published a Health Advisory stating that mpox Vaccines are expected to be effective for both Clade I and Clade II infections. However, as the European CDC recently reported, real-world data regarding the effectiveness of the JYNNEOS vaccine against Clade 1 is lacking.

A new framework was recently released by the World Health Organization (WHO) that will guide health authorities, communities, and other stakeholders in preventing and controlling mpox outbreaks, eliminating human-to-human transmission of the disease, and reducing spillover of the virus from animals to humans.
Mpox is a viral illness caused by the monkeypox virus (MPXV).
There are two different clades of the MPXV: clade I and clade II. Clade I outbreaks are deadlier than clade II outbreaks.
In the United States, clade II cases were reported to have a fatality rate of .002%.
A significant outbreak of the clade I virus in the Democratic Republic of the Congo (DRC) continues today, where cases have been detected for decades. Since the beginning of 2024, over 6,500 cases and 345 deaths have been reported in the DRC.
This data reflects a fatality rate of .05%.
As of May 26, 2024, most U.S. mpox cases continue to be in people who are not vaccinated or have only received one dose of the JYNNEOS® (MVA-BN®, IMVAMUNE®) vaccine. The U.S. CDC recommends that persons at risk for mpox exposure complete the 2-dose vaccination.
In the U.S., the JYNNEOS vaccine is offered at clinics and pharmacies in select cities.

New York City's Deputy Commissioner, Division of Disease Control, Celia Quinn, MD, MPH, issued a statement confirming that mpox continues circulating in the city.
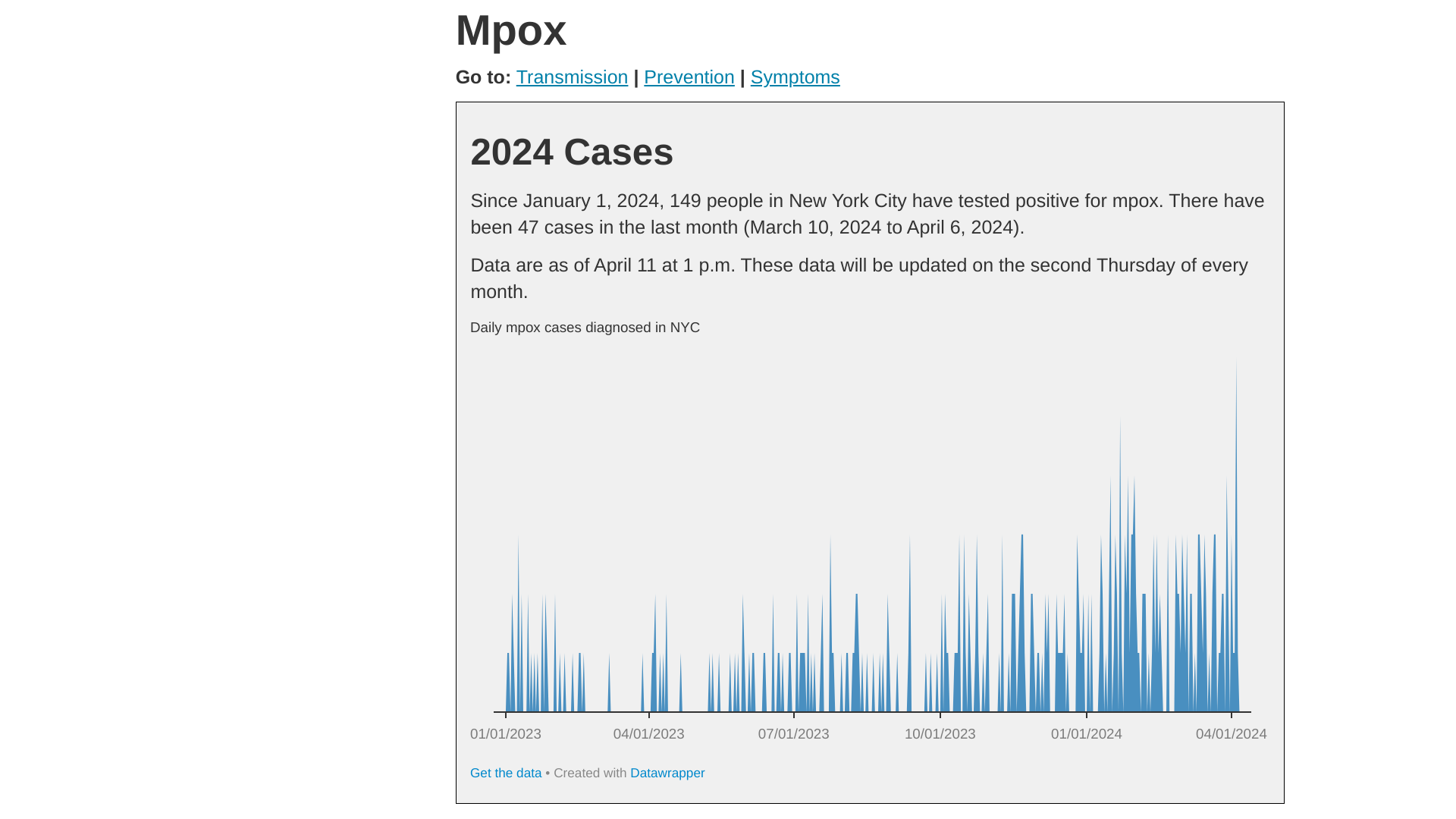
As of May 3, 2024, Health Advisory #12 reported the overall number of mpox cases is low compared to the 2022 outbreak. For most of 2023, the NYC averaged about 2 to 20 monthly cases.
Since October, there has been an average of 36 monthly cases, with a peak count of 51 in January 2024.
Of the 256 cases from October 2023 through April 15, 2024:
- 73% (188) were not vaccinated or had received only one dose,
- 94% were among men,
- 3.9% (10) of the infected people were hospitalized,
- Most were Black or Hispanic and between the ages of 25-44.
NYC confirmed the commercialization of the U.S. FDA-approved JYNNEOS® Mpox Smallpox vaccine is underway. As of May 2024, healthcare providers can order JYNNEOS through their distribution partners to make it available for at-risk individuals at local pharmacies.
Mpox is a contagious disease caused by the monkeypox virus. The U.S. Centers for Disease Control and Prevention and NYC continue to report only Clade II mpox cases in 2024.
Last week, the U.S. Government Accountability Office noted in a May 2, 2024 report that the Strategic National Stockpile (SNS) wasn't clear on how and from whom to request mpox supplies, including causing confusion and delays in mpox vaccine deliveries.
About 30% of U.S. jurisdictions during the mpox outbreak said the process for requesting SNS inventory did not follow written guidelines.
The U.S. Centers for Disease Control and Prevention (CDC) today announced a Health Alert Network (HAN) Health Advisory about the occurrence, geographic spread, and sexually associated human-to-human transmission of Clade I Monkeypox virus (MPXV) in the Democratic Republic of the Congo (DRC).
Since January 2023, the DRC has reported 12,569 suspected mpox cases and 581 related deaths from 22 regions.
The new HAN says cases of Clade I MPXV have not been reported in the United States as of December 7, 2023. The global outbreak of Clade II MPXV was initially reported in May 2022.
However, clinicians should be aware of the possibility of Clade I MPXV in travelers who have been in DRC.
Third-party data indicate that the number of tourists arriving in the DRC was about 460,880 in 2021.
The CDC recently issued a Travel Health Notice (Level 2 - Practice Enhanced Precautions) for people traveling to DRC. Furthermore, there are no direct commercial passenger flights from DRC to the U.S. as of December 2023.
U.S. FDA-approved vaccines (JYNNEOS, ACAM2000) are expected to be effective for both Clade I and II MPXV infections.
Vaccination or prior MPXV infection should provide antibodies that will provide cross-protection to other orthopoxviruses, including Clade I MPXV, says the CDC.
However, clinical verification is under review.
The CDC recommends clinicians encourage vaccination for eligible patients.
Eligible patients who have only received one dose of Bavarian Nordic JYNNEOS® (MVA-BN®, IMVAMUNE®) vaccine, which is based on a live, attenuated vaccinia virus, should receive the second dose as soon as possible, regardless of the time that has elapsed since the first dose.
Mpox vaccines have limited availability in the U.S.
Furthermore, clinicians should notify their state health department if they have a patient with mpox-like symptoms and should submit lesion specimens for clade-specific testing for these patients.