Search API
The U.K. Health Security Agency (UKHSA) recently announced the risk of Middle East Respiratory Syndrome (MERS-CoV) infection to U.K. residents traveling to the Middle East remains 'very low.'
As of June 28, 2022, the National Travel Health Network and Centre and the World Health Organization (WHO) do not advise any travel restrictions to the Kingdom of Saudi Arabia (KSA) concerning MERS-CoV.
MERS-CoV can be spread person-to-person if there is close contact with an infected person, so it is essential to practice good hand and respiratory hygiene, says the UKHSA.
Camels are a source of MERS-CoV infection, so avoiding contact with them is advised.
The WHO reported as of May 12, 2022, the total number of laboratory-confirmed MERS-CoV infection cases reported globally was 2,591, including 894 associated fatalities.
Most of the reported MERS-Cov cases have occurred in countries in the Arabian Peninsula.
Outside this region, there was one large outbreak in the Republic of Korea in May 2015, during which 186 laboratory-confirmed cases and 38 related fatalities were reported.
The UKHSA stated yesterday 'We urge pilgrims returning from Hajj and Umrah to look out for these MERS-CoV symptoms; fever, coughing, shortness of breath, or difficulty breathing. If individuals experience these symptoms within 14 days of leaving the KSA, they should speak with a healthcare provider and mention their travel history.
Dr. Gavin Dabrera, lead for MERS-CoV at UKHSA, said in a media release, "We strongly advise travelers to avoid contact with camels and consumption of camel products in the KSA and to practice good hand hygiene."
As of June 29, 2022, the U.S. FDA had not approved a MERS-CoV prevention vaccine, but several are conducting clinical trials.
Additional Hajj and Umrah vaccine requirements are posted at Vax-Before-Travel.com/Hajj.
Note: This information was manually curated for travelers.

India-based Gennova Biopharmaceuticals Ltd. announced today that its GEMCOVAC™-19 mRNA vaccine received the Emergency Use Authorization from the office of the Drugs Controller General of India.
GEMCOVAC-19 is the first mRNA vaccine developed in India and the only third mRNA vaccine approved for COVID-19 worldwide.
The U.S. FDA has already approved two mRNA vaccines for infants, adolescents, and adults.
GEMCOVAC-19 had reached the primary endpoint of the Phase III clinical trial, and the Central Drugs Standard Control Organisation evaluated the data.
The vaccine was found to be safe, well-tolerated, and immunogenic.
In addition, mRNA vaccines are considered safe as they are deemed non-infectious, non-integrating in nature, and degraded by standard cellular mechanisms.
Notably, this technology provides flexibility to quickly tweak the vaccine for any existing or emerging virus variants, and this technology platform will empower India to be pandemic ready, says Gennova.
Gennova aims to produce around 40 – 50 lakhs of doses per month, and this capacity can be quickly doubled.
Beyond India, Gennova aims to provide sustainable access to low-and middle-income countries.
Gennova, headquartered in Pune, India, is a biotechnology company dedicated to developing, producing, and commercializing biotherapeutics to address life-threatening diseases across various indications.
Note: This press release was manually curated for mobile readers.

The European Commission (EC) announced today that the Health Emergency Preparedness and Response Authority (HERA) is responding to the expanding monkeypox virus outbreak with an initial delivery of 5,300 IMVANEX® vaccine doses in Spain.
As of June 28, 2022, HERA purchased 109,090 monkeypox vaccine doses.
The EC stated this is the first of a series of vaccine deliveries that will take place in the coming weeks to ensure that all Member States are ready to respond to the monkeypox outbreak, which has exceeded 4,400 cases globally since early May 2022.
As was the case with COVID-19 vaccines, IMVANEX vaccines will be allocated on a pro-rata basis, according to the population of each country.
EC Commissioner for Health and Food Safety Stella Kyriakides commented in a press release, "As of today, the first deliveries of vaccines in response to the monkeypox outbreak are arriving in the most affected countries."
Portugal, Germany, and Belgium will be the next countries to receive vaccine doses.
"The European Health Union responds in real-time to new health threats and protects its citizens."
"This is the first time that we are, through our HERA, directly buying and donating vaccines to Member States."
It is also the first time the EU budget was used to purchase vaccines donated directly to Member States.
"With HERA up and running, the EU has significantly reinforced its capacity to respond and address new health threats decisively, added Kyriakides.
In its recent risk assessment, the European Centre for Disease Control and Prevention advised affected countries to consider early post-exposure vaccination, using the 3rd generation vaccine produced by Bavarian Nordic, to prevent the contagious monkeypox disease.
In the USA, the Jynneos vaccine distribution is managed by the U.S. CDC.
However, according to New York City and Washington DC reports, consumer demand is overwhelming Jynneos vaccine availability.
Eligible New Yorkers who may have been recently exposed to the monkeypox virus can get the two-dose vaccine at the Chelsea Sexual Health Clinic.
Unfortunately, NYC Health's vaccine supply has been exhausted.
And in Washington, DC, Mayor Bowser and DC Health announced a limited amount of monkeypox vaccination appointments would become available to eligible District residents on June 27, 2022.
But, DC Health's website now says, 'All monkeypox vaccine appointments have been scheduled.'
'Residents should check DC Health's social platforms on Wednesday (6/29) to get an update on additional appointment availability.'
Furthermore, based on the rapid increase in monkeypox cases in the USA, 306 patients in twenty-six states as of today, vaccine demand will continue increasing.
To date, the U.S. government has received requests from 32 states and jurisdictions, deploying over 9,000 doses of vaccine.
The United States announced late on June 28, 2022, that it intends to allocate 296,000 vaccine doses over the coming weeks, 56,000 of which will be allocated immediately.
And in the coming months, a combined 1.6 million additional doses will become available.
Additional monkeypox outbreak news is posted at Vax-Before-Travel.
Note: This information was converted into English and manually curated for mobile readership.

The U.S. CDC announced today it continues to deploy an aggressive public health response to the monkeypox outbreak in the USA by activating its Emergency Operations Center (EOC).
This action stands up the EOC for monitoring and coordinating the emergency response to monkeypox and mobilizing 300 additional CDC personnel and resources.
The activation of the EOC will further supplement the ongoing work of the CDC in collaboration with local, national, and international response partners.
As of June 27, 2022, the CDC confirmed about twenty-four states had reported 224 monkeypox patients.
Internationally, about 79 countries have confirmed over 4,400 monkeypox cases.
Last week, CDC began shipping orthopoxvirus tests to five commercial laboratory companies, including the nation’s largest reference laboratories, to increase monkeypox testing capacity and access in every community.
In June 2022, the CDC updated and expanded the monkeypox case definition and continues encouraging health care providers to consider testing for all rashes with clinical suspicion of monkeypox.
Health care providers who see a patient with a rash that resembles monkeypox or might be more characteristic of more common infections (e.g., varicella-zoster, herpes zoster, or syphilis) should carefully evaluate the patient for monkeypox and should consider testing.
Anyone with risk factors for monkeypox and a new rash should seek care and testing.
Additional monkeypox outbreak breaking news is posted on this weblink.

Massachusetts-based VBI Vaccines Inc. announced today that new data from a follow-up analysis of a subset of participants from the pivotal Phase 3 study of the Company's 3-antigen prophylactic hepatitis B (HBV) vaccine known in the USA as PreHevbrio™.
Timo Vesikari, M.D., Ph.D., Professor Emeritus and Director of the Nordic Vaccine Research Network in Finland, and principal investigator of the PROTECT and CONSTANT Phase 3 clinical studies, highlighted data from his investigator-initiated analysis that evaluated the duration of immune response approximately 2.5 years after completion of vaccination.
In the follow-up analysis, participants in PROTECT who received VBI's 3-antigen HBV vaccine had 5.5-fold higher mean anti-HBs titers (GMC: 1382.9 mIU/mL vs. 251.4 mIU/mL) and a higher seroprotection rate (SPR: 88.1% vs. 72.4%) compared to those who received Engerix-B, another vaccine.
Additionally, 72.9% of participants who received VBI's 3-antigen HBV vaccine retained anti-HBs titers ≥ 100 mIU/mL compared to 32.6% of those who received Engerix-B.
VBI's hepatitis B vaccine is the only 3-antigen hepatitis B vaccine, comprised of the three surface antigens of the hepatitis B virus – S, pre-S1, and pre-S2.
It is approved for use in the U.S. for adults and in the European Union/European Economic Area, United Kingdom, and Israel.
Hepatitis B is one of the world's most significant infectious disease threats, with more than 290 million people infected globally, says the U.S. CDC.
In addition, an estimated 887,000 people die each year from complications of chronic HBV.
The CDC says most hepatitis viruses can be prevented by vaccination.
However, the five hepatitis viruses – hepatitis A, hepatitis B, hepatitis C, hepatitis D, and hepatitis E – are distinct and can spread differently.
HBV is transmitted by contact with contaminated blood, blood products, and other body fluids.
Examples of exposures associated with a transmission that travelers may encounter include poor infection control during medical or dental procedures, receipt of blood products, injection drug use, tattooing or acupuncture, and unprotected sex.
HBV infection is the leading cause of liver disease, and with current treatments, it is challenging to cure, with many patients developing liver cancers.
Additional hepatitis vaccine news is posted at PrecisionVaccinations.com/Hepatitis.
Note: VBI's press release was manually curated for mobile readership.

Tuberculosis (TB) continues to be a significant public health problem worldwide, generally prevented in developing countries with the 100-year-old BCG vaccine. Yet, despite being a preventable and curable disease, 1.5 million people die from TB each year, making it the world's top infectious killer.
Over the years, immunization with the Mycobacterium bovis BCG vaccine has partially protected infants' lungs, reducing the incidence of miliary and tuberculosis meningitis.
However, the BCG vaccine is less effective against pulmonary TB.
A global team of researchers aimed to compare the safety and immunogenicity of Serum Institute of India (SSI) Pvt. Ltd.'s VPM1002 recombinant BCG vaccine candidate with BCG in HIV exposed and HIV unexposed newborn babies.
This study is essential since TB is the leading cause of death of people with HIV and also a major contributor to antimicrobial resistance, says the World Health Organization (WHO).
The results from the double-blind, randomized, active-controlled phase 2 study conducted at health centers in South Africa were published in the peer-review journal The Lancet Infectious Diseases on June 27, 2022.
Participants were randomly assigned to VPM1002 or BCG vaccination in a 3:1 ratio, stratified by HIV status. And the primary outcome was assessed in all vaccinated participants (safety population) at regular follow-up visits until 12 months after vaccination.
These researchers concluded both vaccines were immunogenic in these children, although responses were higher with the old BCG vaccine.
But the VPM1002 vaccine was found less reactogenic than BCG and was not associated with any serious safety concerns.
The VPM1002 vaccine is a genetically modified BCG vaccine derived from the Mycobacterium Bovis BCG subtype Prague characterized as rBCGÄureC::Hly+.
VPM1002 contains weakened tuberculosis-like bacteria, genetically modified, empowering immune cells to recognize the bacteria better.
SSI is currently funding a multicentric phase 3 clinical trial in babies in sub-Saharan Africa with VPM1002.
The WHO says about one-quarter of the world's population is estimated to be infected by TB.
About half of all people with TB can be found in just eight countries: Bangladesh, China, India, Indonesia, Nigeria, Pakistan, Philippines, and South Africa.
TB vaccination is not standard for children in the USA since most reported cases are travel-related.
As of June 28, 2022, the U.S. FDA had issued Organon Teknika Corp., LLC Approvals for two BCG vaccines: BCG Live and TICE BCG.
The WHO's Global Tuberculosis Report was last published on October 14, 2021.
Note: This study's findings were manually curated for mobile readership.

In response to the ongoing meningococcal outbreak in Florida, the Florida Department of Okaloosa County recently announced it is now providing meningococcal vaccinations at no-cost to adults.
As of June 24, 2022, the activity in 2022 surpasses Florida's 5-year average of meningococcal disease cases.
This is a serious disease caused by bacteria called Neisseria meningitidis.
At least seven related fatalities and 24 cases of the disease have been reported in 2022.
Fortunately, these bacteria are not as contagious as germs that cause the common cold or flu.
People do not catch the bacteria through casual contact or breathing the air where someone with meningococcal disease has been.
Instead, it requires close contact over a period of time or direct contact such as kissing or sharing drinks.
The two most common types of meningococcal infections are meningitis (an infection of the lining of the brain and spinal cord) and bloodstream infection, both of which can quickly become deadly.
"Getting vaccinated against meningococcal disease is the best way to prevent this serious illness, which can quickly become deadly," said José R. Romero, M.D., Director, National Center for Immunization and Respiratory Diseases, in a media statement issued on June 22, 2022.
"Because of the outbreak in Florida, and the number of Pride events being held across the state in coming weeks, it's important those traveling to Florida talk to their healthcare provider about getting a MenACWY vaccine."
The Florida Health Department suggests the following groups should consider vaccination with a meningococcal conjugate (MenACWY) vaccine during this outbreak:
- Men who have sex with men ages 18 and older
- People living with HIV ages 18 and older
- LGBTQ+ ages 18 and older
- Immunocompromised individuals ages 18 years and older
- People in the above groups received their MenACWY vaccine more than five years ago.
Leon County, FL, also reported an unrelated serogroup B meningococcal disease cluster among college and university students in May 2022.
And as of June 26, 2022, the U.S. CDC had not issued an official health alert about these meningococcal outbreaks in Florida.
However, the CDC's vaccine committee updated its recommendations for meningococcal vaccines on June 23, 2022.
Note: This information was manually curated for mobile travelers.

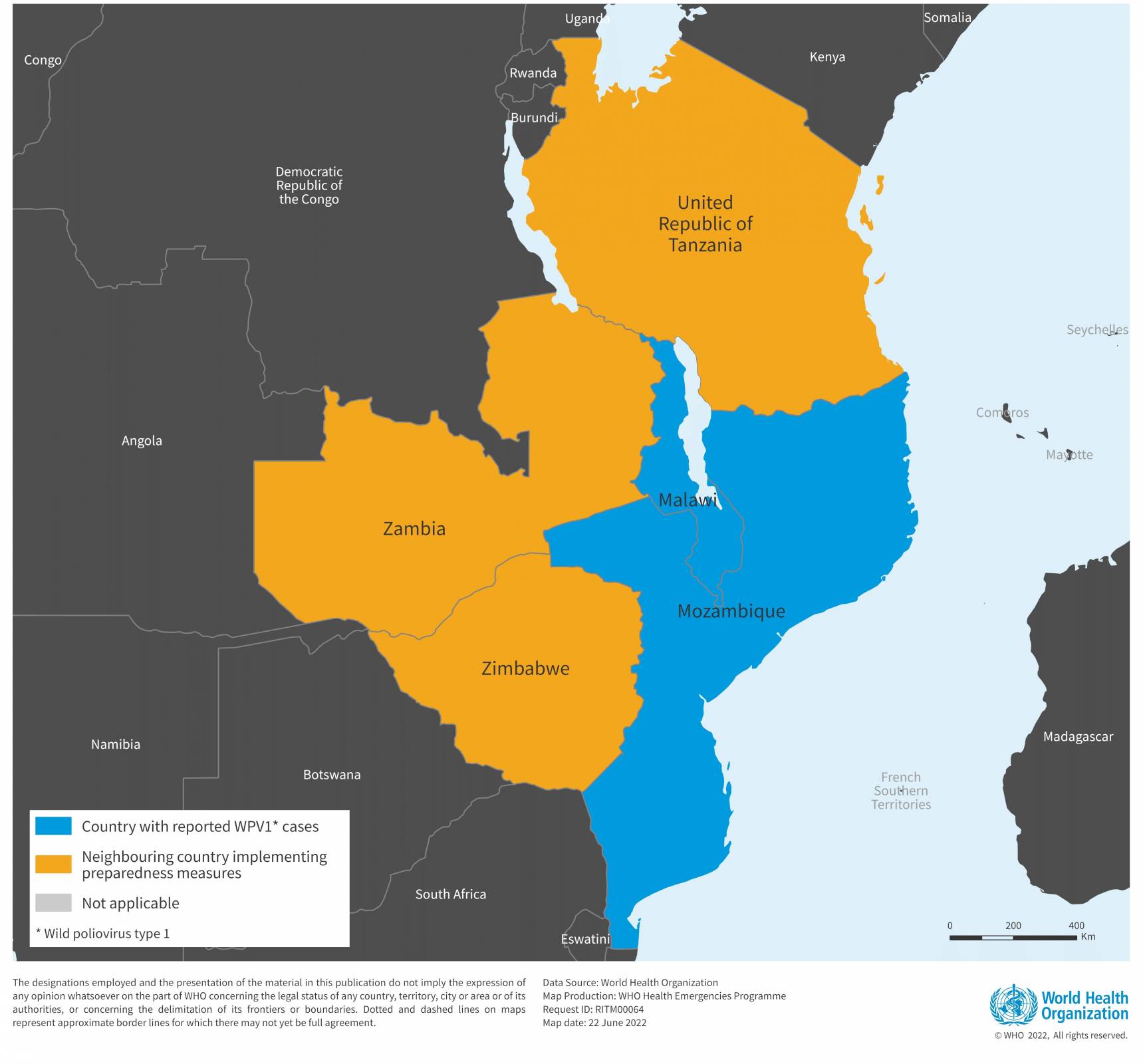
The World Health Organization (WHO) recently reported details on a wild poliovirus type 1 (WPV1) case in Mozambique that is genetically linked to a strain detected in Pakistan and similar to a case confirmed in Malawi during February 2022.
Mozambique is also affected by a concurrent outbreak of circulating vaccine-derived poliovirus type 2 (cVDPV2), with seven cases reported since 2021 and is ongoing.
Mozambique has been actively participating in the multi-country emergency outbreak response implemented across the South East Africa region in response to the case of WPV1 reported in Malawi, alongside Tanzania, Zambia, and Zimbabwe, to reach more than 23 million children across the region.
Two rounds of bivalent OPV vaccination campaigns have already been implemented, the most recent at the end of April 2022, with more than 4.5 million children vaccinated in Mozambique.
According to the WHO-UNICEF national immunization coverage estimate, the oral poliovirus vaccine third dose (OPV3) and inactivated poliovirus vaccine first dose (IPV1) coverage was 73% and 78%, respectively, in Mozambique in 2020.
The WHO stated on June 23, 2022, 'The risk of international spread, particularly across the South East region of Africa, remains high, due to persisting sub-optimal immunity and surveillance gaps, and large-scale population movements.'
The WHO considers that there is a continuous high risk of international spread of WPV1, and the risk associated with the concurrent cVDPV2 outbreak is currently assessed as moderate.
Poliomyelitis (polio) is a highly infectious viral disease that predominantly affects children under five.
The virus is shed by infected people (usually children) through feces, where it can spread quickly, especially in areas with poor hygiene and sanitation systems.
However, the developed countries of Isreal and the U.K. have both confirmed polio in wastewater samples in 2022.
In response, Israel launched a substantive polio vaccination campaign in April 2022, hoping to reach millions of children.
To inform international travelers about their potential polio risk, the U.S. CDC issued Alert - Level 2, Practice Enhanced Precautions notices for countries in Africa, Asia, and Eastern Europe in March 2022.
The CDC says, 'Before any international travel, anyone unvaccinated, incompletely vaccinated, or with an unknown polio vaccination status should complete the routine polio vaccine series.'
And 'adults who previously completed the full, routine polio vaccine series, have not yet received an adult booster dose, and are going to any of the destinations listed above, should receive a single, lifetime booster dose of a polio vaccine,' says the CDC.
There are several polio vaccines authorized globally, which are listed at Vax-Before-Travel.com/Polio.
Bacteria causing Typhoid fever are becoming increasingly resistant to some of the most important antibiotics for human health, according to a new study published in The Lancet Microbe journal on June 21, 2022.
Typhoid fever is a global public health concern, causing 11 million infections and more than 100,000 deaths yearly.
While it is most prevalent in South Asia – which accounts for 70% of the global disease burden – it also has significant impacts in sub-Saharan Africa, Southeast Asia, and Oceania, highlighting the need for a worldwide response.
Lead author, Dr. Jason Andrews, Stanford University, commented in a related press release, "The speed at which highly-resistant strains of S. Typhi have emerged and spread in recent years is a real cause for concern and highlights the need to urgently expand prevention measures, particularly in countries at greatest risk."
'We found frequent international (n=138) and intercontinental transfers (n=59) of antimicrobial-resistant S Typhi, followed by local expansion and replacement of drug-susceptible clades.
"At the same time, the fact resistant strains of S. Typhi have spread internationally so many times also underscores the need to view typhoid control, and antibiotic resistance more generally, as a global rather than local problem."
The findings add to recent evidence of the rapid rise and spread of S. Typhi strains resistant to third-generation cephalosporins, another class of antibiotics critically important for human health.
To help protect international travelers, the U.S. FDA has approved two typhoid vaccines.
And the U.S. CDC published a Watch - Level 1, Practice Usual Precautions for Pakistan in 2021 to alert visitors to the region.

